Preparation method of 3, 5-dichlorobenzaldehyde and carboxamide triazole intermediate
A technology of dichlorobenzyl alcohol and carboxyamine triazole is applied in the field of pharmaceutical synthesis, can solve the problems of complicated separation and purification steps, low safety, many residues, etc., and can meet the requirements of pharmaceutical production quality, industrialized large-scale production, and production safety. The effect of high performance and overcoming the difficulty of temperature control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
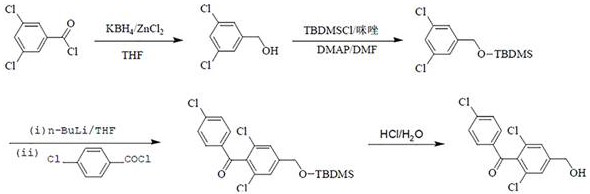
[0033] The invention provides a kind of preparation method of 3,5-dichlorobenzyl alcohol, comprising the steps of:
[0034] Mix 3,5-dichlorobenzoyl chloride, potassium borohydride and zinc chloride in a solvent, heat to reflux for reduction reaction; concentrate after the reduction reaction to prepare crude product;
[0035] Add hydrochloric acid aqueous solution and toluene to the crude product for extraction or add toluene for extraction, collect the toluene phase, wash with alkaline aqueous solution and water until neutral, and then concentrate to dryness, and the obtained solid is reconstituted with n-hexane or n-heptane crystallization.
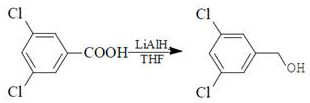
[0036] The preparation method of the above-mentioned 3,5-dichlorobenzyl alcohol uses 3,5-dichlorobenzoyl chloride as a raw material, and carries out 3,5-dichlorobenzoyl chloride by using potassium borohydride and zinc chloride compatibility as a reducing system. Reduction, avoiding the use of LiAlH in traditional industrial production ...
Embodiment 1
[0057] The present embodiment is a kind of preparation method of carboxyamine triazole intermediate, and the steps are as follows:
[0058] (1) Add 20 kg of zinc chloride, 300 kg of THF, and 20 kg of potassium borohydride into the reaction pot, heat to 45°C and reflux for 2 hours, and add 55 kg of 3,5-dichlorobenzoyl chloride dropwise while maintaining the reflux state, Then rinse the feeding tube with a small amount of THF, heat to reflux at 45°C and keep for 3 hours. Then evaporate THF to dryness, cool to below 60°C, add 10 kg of water dropwise, then add water to 150 kg, add 3 kg of hydrochloric acid, 180 kg of toluene at the same time, separate layers and wash the toluene solution once, and use sodium carbonate aqueous solution (2 kg of sodium carbonate Add 150 kg of water to dissolve it) wash the toluene solution once, and wash with water until neutral. Evaporate toluene to dryness under reduced pressure at 100°C, add 50 kg of n-hexane after slight cooling, discharge and ...
Embodiment 2
[0067] This example is a preparation method of a carboxyamine triazole intermediate, the steps are the same as in Example 1, the difference is that in step (1) n-heptane is used instead of n-hexane, and in step (3) methyl tert-butyl ether is used Substitute for isopropyl ether.
[0068] Specific steps are as follows:
[0069] (1) Add 20 kg of zinc chloride, 300 kg of THF, and 20 kg of potassium borohydride into the reaction pot, heat to 45°C and reflux for 2 hours, and add 55 kg of 3,5-dichlorobenzoyl chloride dropwise while maintaining the reflux state, Then rinse the feeding tube with a small amount of THF, heat to reflux at 45°C and keep for 3 hours. Then evaporate THF to dryness, cool to below 60°C, add 10 kg of water dropwise, then add water to 150 kg, add 3 kg of hydrochloric acid, 180 kg of toluene at the same time, separate layers and wash the toluene solution once, and use sodium carbonate aqueous solution (2 kg of sodium carbonate Add 150 kg of water to dissolve), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com