Triarylamine derivative fluorescent probe as well as preparation method and application thereof
A technology of fluorescent probe and triarylamine, which is applied in the field of triarylamine derivative fluorescent probe and its synthesis, and achieves the effects of obvious detection effect, specificity and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
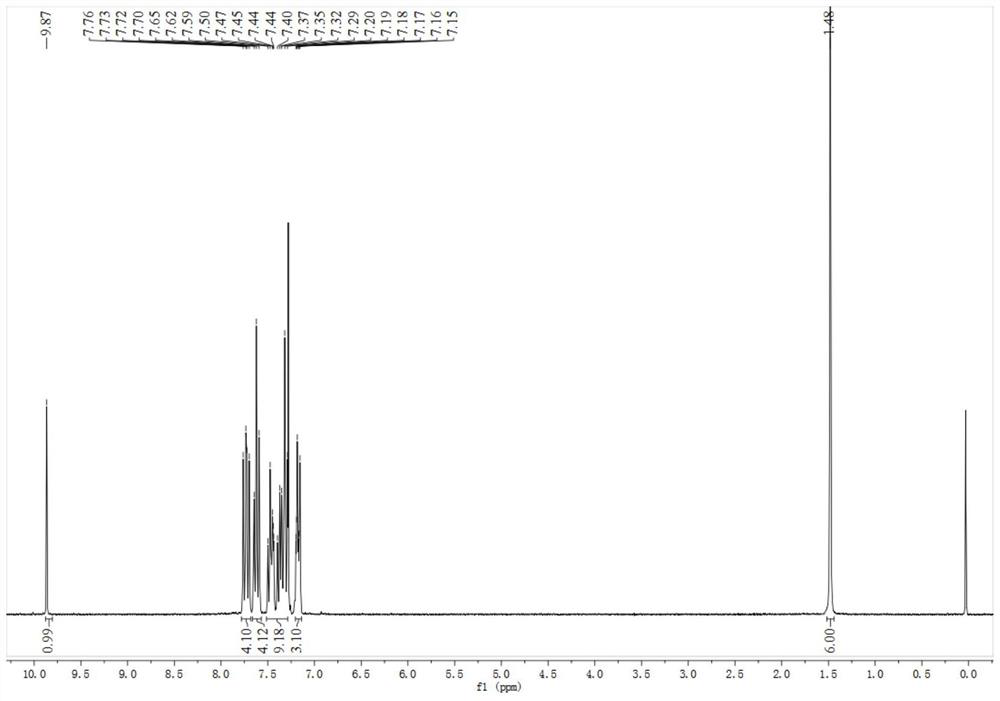
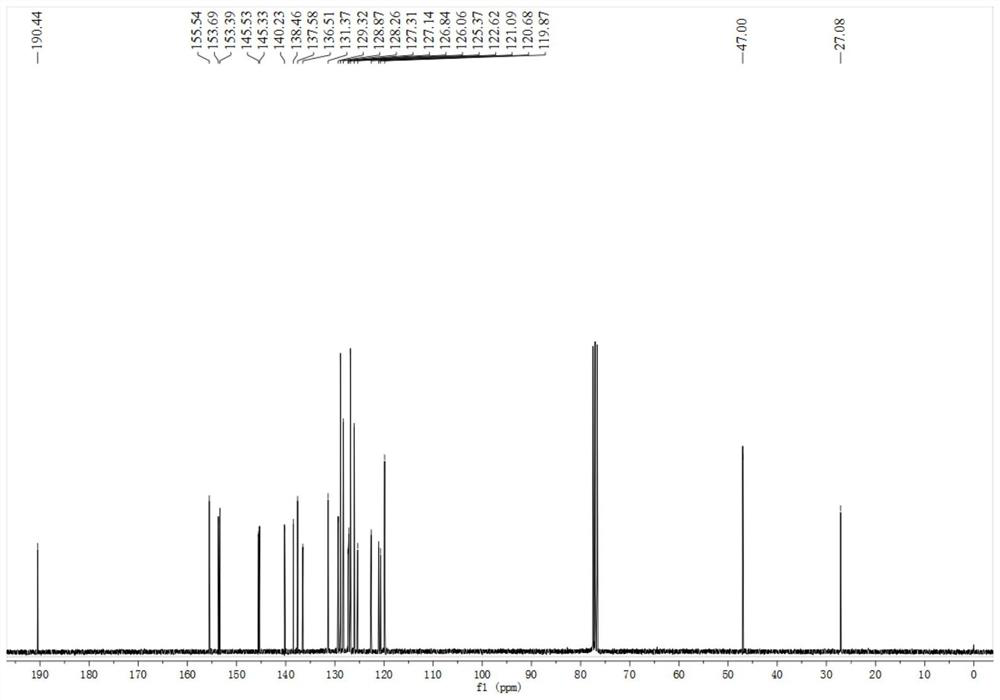
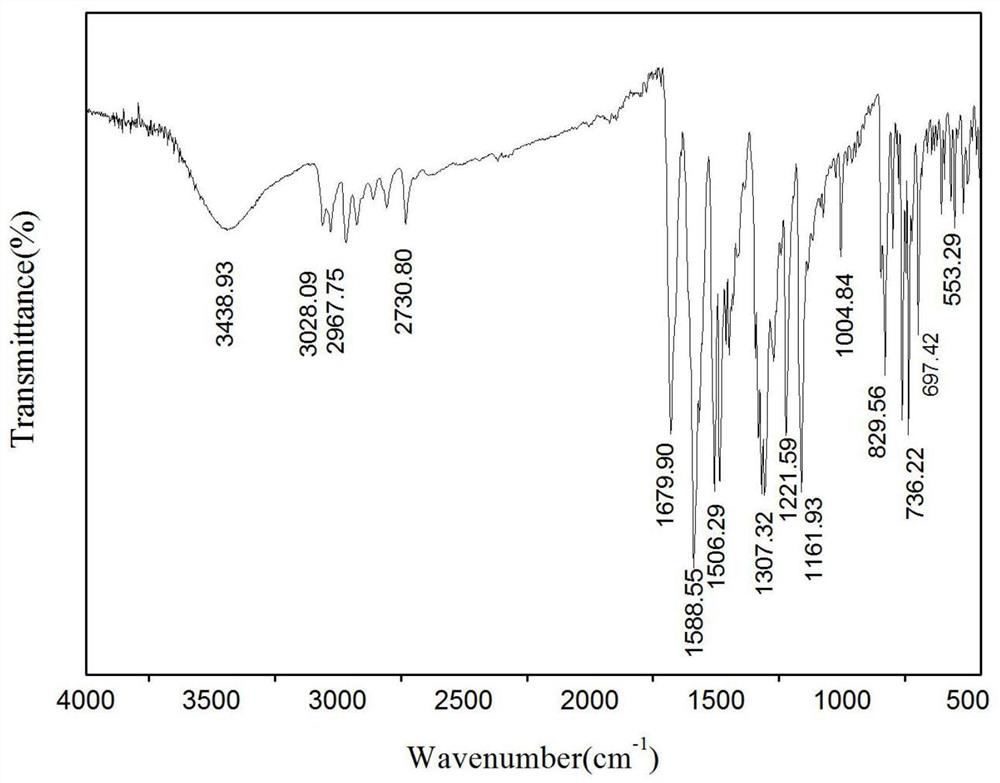
[0040] Specific embodiment one: The structural formula of the triarylamine derivative fluorescent probe in this embodiment is as follows:
[0041]
specific Embodiment approach 2
[0042] Specific embodiment two: the synthesis method of triarylamine derivative fluorescent probe is as follows:
[0043] (1): N 2 protection, anhydrous toluene as solvent, under reflux conditions, cesium carbonate, tri-tert-butylphosphine, Pd 2 DBA 3 Under catalysis, N-(biphenyl-4-yl)-9,9-dimethyl-9H-fluorene-2-amine and p-chlorobenzaldehyde undergo a coupling reaction, the organic phase is washed with water, dried, concentrated, and the column Chromatography (PE:EA=10:1) separation gave triarylamine intermediate. The molar ratio of N-(biphenyl-4-yl)-9,9-dimethyl-9H-fluoren-2-amine to p-chlorobenzaldehyde is 1:1.
[0044] (2): Under the action of dichloromethane as a solvent and boron trifluoride ether, the triarylamine intermediate reacts with ethanethiol at room temperature, the organic phase is washed with water, dried, concentrated, and subjected to column chromatography (PE:EA=12:1) Get fluorescent probes.
specific Embodiment approach 3
[0045] Specific embodiment three: the difference between this embodiment and specific embodiment two is: the reaction molar ratio of step (2) triarylamine intermediate and ethanethiol is 1:2.5. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com