Graphene oxide supported ruthenium catalyst as well as preparation method and application thereof
A ruthenium catalyst and supported technology, which is applied in the direction of physical/chemical process catalysts, catalytic reactions, carbon-based compound preparation, etc., can solve the problems of harsh reaction conditions and many by-products, and achieve the effect of simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 graphene oxide supported ruthenium catalyst
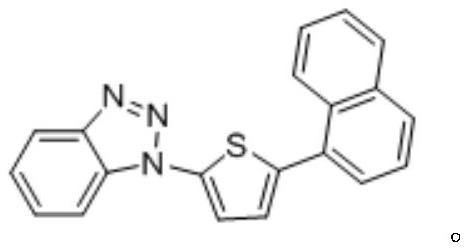
[0048] (1) Put 10mmol of benzotriazole, 15mmol of 2,5-dibromothiophene, and 7.5mmol of potassium tert-butoxide in a 100mL round bottom flask, add 30mL of toluene to it, place it in an oil bath at 125°C, and stir it magnetically React for 24 hours. After the reaction is completed, wait for it to cool to room temperature, collect the filtrate by suction filtration, and remove the solvent by rotary evaporation of the filtrate. Add 10 mmol 1-naphthylboronic acid, 3 mmol palladium chloride, 8 mmol potassium phosphate and 40 mL distilled water to the residue obtained. React at 110°C for 12 hours. After the reaction, extract with ethyl acetate three times, collect the organic phase, and remove the solvent by rotary evaporation to obtain 3.12 g of solid. In the presence of nitrogen, add 3.12 g of solids and 1.6 g of ruthenium trichloride into the Schlenk bottle, reflux reaction in ethylene glycol methyl ether at 120 ° C...
Embodiment 2
[0051] Embodiment 2 graphene oxide supported ruthenium catalyst
[0052] (1) Put 10mmol of benzotriazole, 17.5mmol of 2,5-dibromothiophene, and 8.5mmol of potassium tert-butoxide in a 100mL round-bottomed flask, add 35mL of toluene to it, place it in an oil bath at 130°C, and magnetically Stir the reaction for 30 hours. After the reaction is completed, wait for it to cool to room temperature, collect the filtrate by suction filtration, and remove the solvent by rotary evaporation of the filtrate. Add 12 mmol 1-naphthylboronic acid, 5 mmol palladium chloride, 10 mmol potassium phosphate and 45 mL distilled water to the obtained residue , reacted at 110°C for 16h, extracted 3 times with ethyl acetate after the reaction, collected the organic phase, and evaporated the organic matter to remove the solvent to obtain 3.84g of solid. In the presence of nitrogen, 3.84 g of solid and 2 g of ruthenium trichloride were added to the Schlenk bottle, and refluxed in ethylene glycol methyl e...
Embodiment 3
[0055] Embodiment 3 graphene oxide supported ruthenium catalyst
[0056] (1) Put 10mmol of benzotriazole, 20mmol of 2,5-dibromothiophene, and 10mmol of potassium tert-butoxide in a 100mL round bottom flask, add 40mL of toluene to it, place it in an oil bath at 140°C, and stir the reaction by magnetic force 36h, after the reaction was completed, wait for it to cool to room temperature, collect the filtrate by suction filtration, and remove the solvent by rotary evaporation of the filtrate, add 15mmol 1-naphthylboronic acid, 8mmol palladium chloride, 10mmol potassium phosphate and 50mL distilled water to the residue obtained, The reaction was carried out at ℃ for 18 hours. After the reaction was completed, it was extracted three times with ethyl acetate, the organic phase was collected, and the organic matter was rotary evaporated to remove the solvent to obtain 4.02 g of solid. In the presence of nitrogen, add 4.02 g of solid and 2.3 g of ruthenium trichloride into the Schlenk ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com