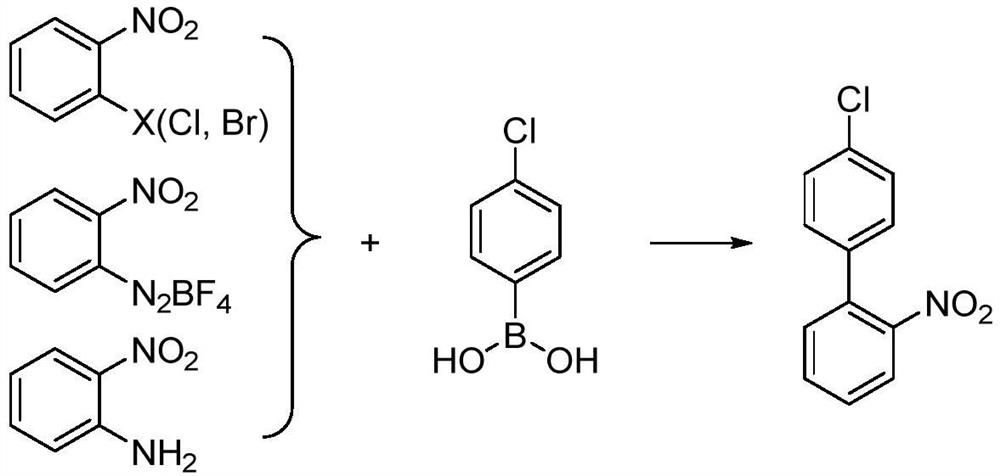
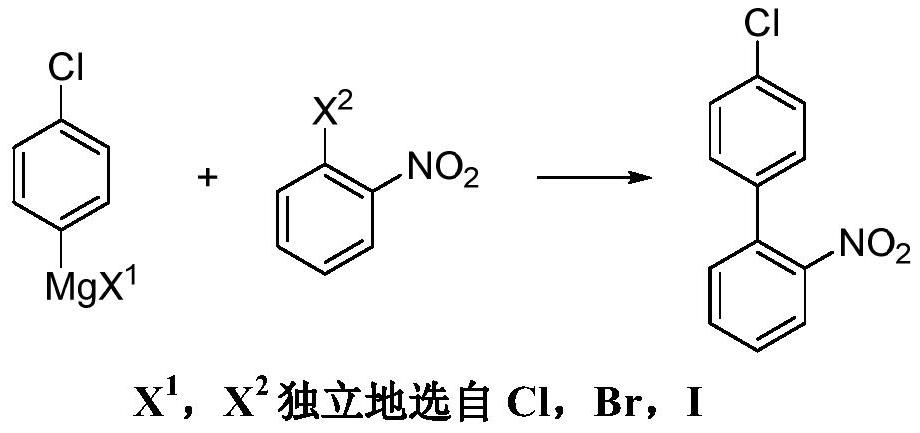
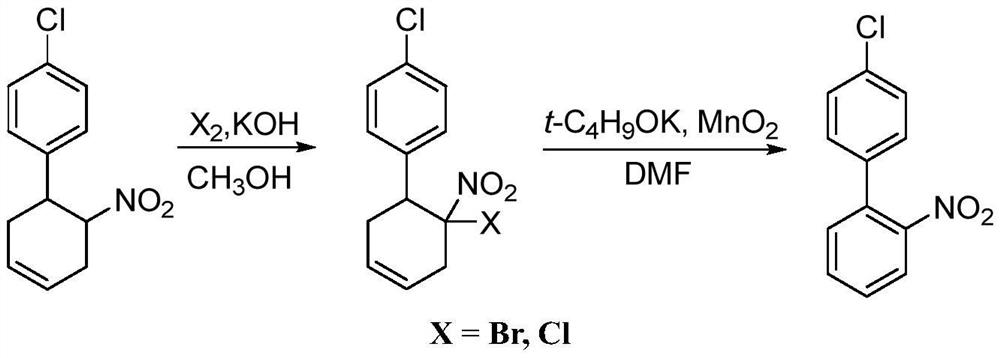
Preparation method of 4'-chloro-2-nitrobiphenyl
A technology of nitrobiphenyl and nitro, applied in the field of organic compound synthesis, can solve the problems of low yield, cumbersome operation, and many "three wastes", and achieve the effect of high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of 4'-chloro-2-nitrobiphenyl of the present invention comprises the following steps:
[0038] Add 23.95g (99%, 0.1mol) 1-chloro-4-(6-nitrocyclohex-3-enyl)-benzene, 26.08g (0.3mol) 1%-Fe / MnO 2and 71.85g of toluene, heated to 110°C and then incubated for 4 hours. After the reaction, the reaction product was filtered, solvent removed, and methanol was added for recrystallization to obtain 19.3 g of yellow solid 4'-chloro-2-nitrobiphenyl with a content of 98.3% and a yield of 81.2%. 1 H-NMR (600MHz, CDCl 3 ): δ=7.23(s, 1H), 7.24(s, 1H), 7.39-7.42(m, 3H), 7.51(d, J=7.8Hz, 1H), 7.61(d, J=5.4Hz, 1H) , 7.87 (d, J=7.8Hz, 1H), demonstrating the successful preparation of 4'-chloro-2-nitrobiphenyl.
[0039] In the present embodiment, the preparation process of oxidizing agent is as follows:
[0040] Weigh 31.6g (0.2mol) of potassium permanganate and dissolve it in 600g of deionized water, slowly add 480g of 30% hydrogen peroxide solution dropwise under stir...
Embodiment 2
[0042] A preparation method of 4'-chloro-2-nitrobiphenyl of the present invention comprises the following steps:
[0043] 23.95g (99%, 0.1mol) 1-chloro-4-(6-nitrocyclohex-3-enyl)-benzene, 34.78g (0.4mol) 0.1%-Co / MnO 2 and 191.6g of chlorobenzene, heated to 128°C and then kept for 5h reaction. After the reaction, the reaction product was filtered, solvent removed, and methanol was added for recrystallization to obtain 19.81 g of yellow solid 4'-chloro-2-nitrobiphenyl with a content of 98.7% and a yield of 83.7%.
[0044] In the present embodiment, the preparation process of oxidizing agent is as follows:
[0045] Weigh 31.6g (0.2mol) of potassium permanganate and dissolve it in 600g of deionized water, and slowly add 480g of 30% hydrogen peroxide solution dropwise under stirring. Use nitric acid and KOH to adjust the reaction system to maintain weak alkalinity (pH=7~9), then add 10g of cobalt nitrate solution containing 0.058g (0.2mmol) dropwise, continue to react for 30min a...
Embodiment 3
[0047] A preparation method of 4'-chloro-2-nitrobiphenyl of the present invention comprises the following steps:
[0048] 23.95g (99%, 0.1mol) 1-chloro-4-(6-nitrocyclohex-3-enyl)-benzene, 17.39g (0.2mol) 5%-Ni / MnO 2 and 95.8g xylene, heated to 138°C and then kept for 6h reaction. After the reaction, the reaction product was filtered, solvent removed, and methanol was added for recrystallization to obtain 20.22 g of yellow solid 4'-chloro-2-nitrobiphenyl with a content of 98% and a yield of 84.8%.
[0049] In the present embodiment, the preparation process of oxidizing agent is as follows:
[0050] Weigh 31.6g (0.2mol) of potassium permanganate and dissolve it in 600g of deionized water, and slowly add 480g of 30% hydrogen peroxide solution dropwise under stirring. Use nitric acid and KOH to adjust the reaction system to maintain weak alkalinity (pH=7~9), then add 10 g of nickel nitrate solution containing 1.83 g (10 mmol) dropwise, continue to react for 30 min after the drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com