Preparation method of lvsong peptide rhzomorph C
A technology of peptidocin and actinomycetes, applied in the field of biopharmaceuticals, can solve the problems of high cost, complex structure, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. The construction of mutant strain DS-1, the construction steps are as follows:
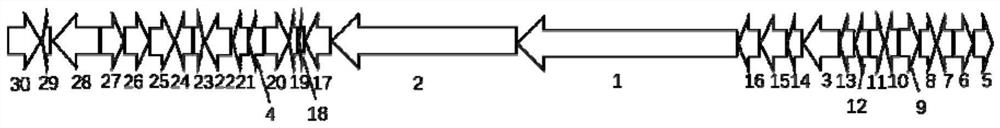
[0045]1. The whole genome of Actinomadura sp. DSM43766 was sequenced to identify the biosynthetic gene cluster of luzonidura. The gene cluster is 48kb long, as shown in SEQ ID NO.1, including 30 genes, such as figure 1 shown.
[0046] Further functional analysis was carried out on 30 genes, and a gene encoding acetylase was determined. The nucleotide sequence of the gene is shown in SEQ ID NO.2, and it was named luz27. The amino acid sequence of the acetylase encoded by the gene is shown in SEQ ID NO.3.
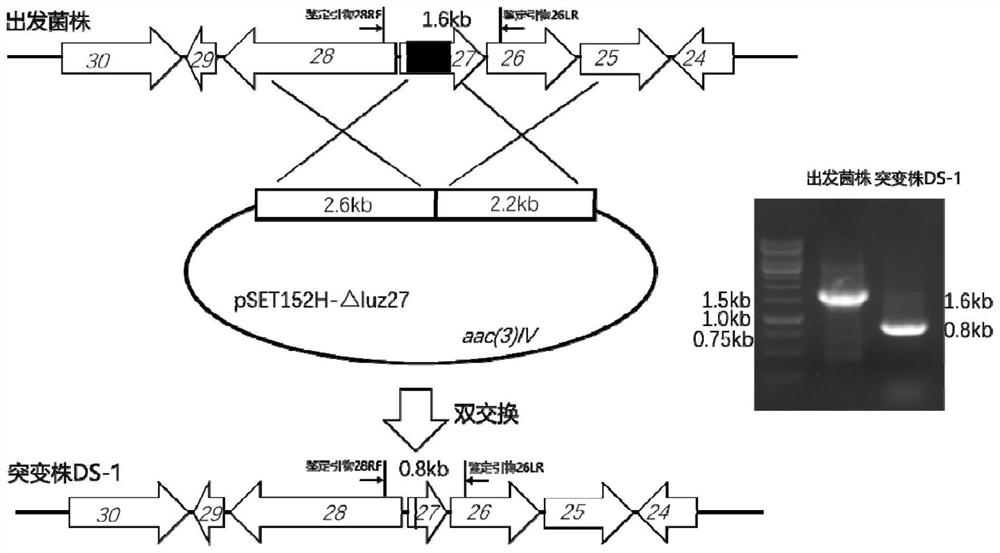
[0047] 2. Based on the Escherichia coli-Streptomyces shuttle plasmid vector pSET152 (Genbank sequence number AJ414670.1), the luz27 gene was knocked out by homologous double crossover method.
[0048] Two homology arms (2.2 kb and 2.6 kb in length, respectively) upstream and downstream of the target knockout region were amplified by polymerase chain reaction using the genomic DNA of ...
Embodiment 2
[0058] Preparation of Luzonin C
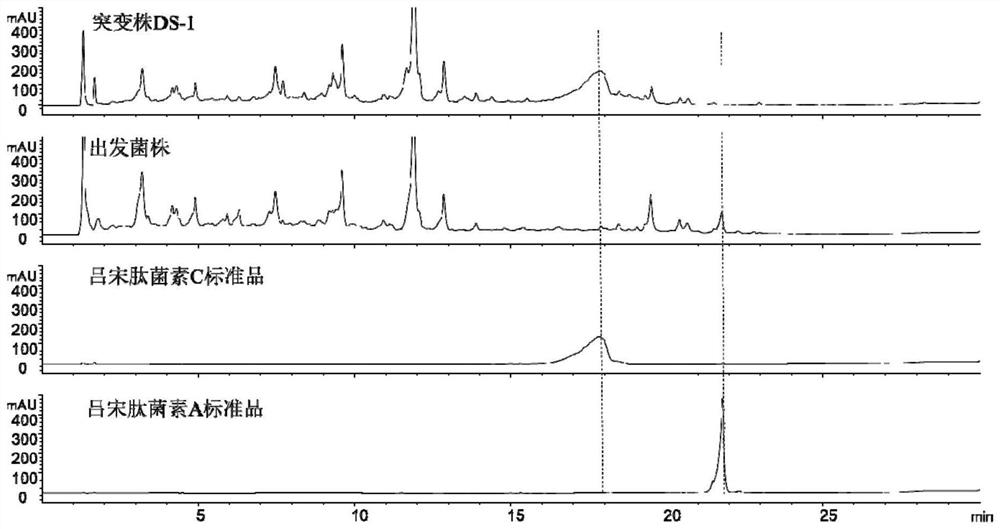
[0059] Actinomadura sp. (Actinomadura sp.) strain DS-1 was cultured in MS medium (1L) for 12 days on a large scale, soaked in ethyl acetate for 3 times, the extracts were combined and filtered, and the ethyl acetate was evaporated to dryness The crude extract was obtained, which was dissolved in 4 ml of methanol and subjected to Sephadex LH20 gel chromatography (pure methanol mobile phase). Aliquot tubes of 5 ml each to receive the eluate. 20 microliters of components in each tube were analyzed with EC-C18 chromatographic column (mobile phase ratio: 0-25min, acetonitrile: water = 10:90-80:20; 25-26min, acetonitrile: water = 80:20~ 100:0; 26-28min, acetonitrile: water = 100:0; 28-29min, acetonitrile: water = 100:0-10:90; 29-30min, acetonitrile: water = 10:90. The mobile phase contains 0.05% Formic acid, the detector is a diode array detector, the flow rate is 1.2mL / min). By comparing with the standard substance of luzopeptide C, find the com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com