Preparation method of supported ruthenium-based catalyst
A ruthenium-based catalyst, supported technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., to achieve the effects of good stability, simple preparation process and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
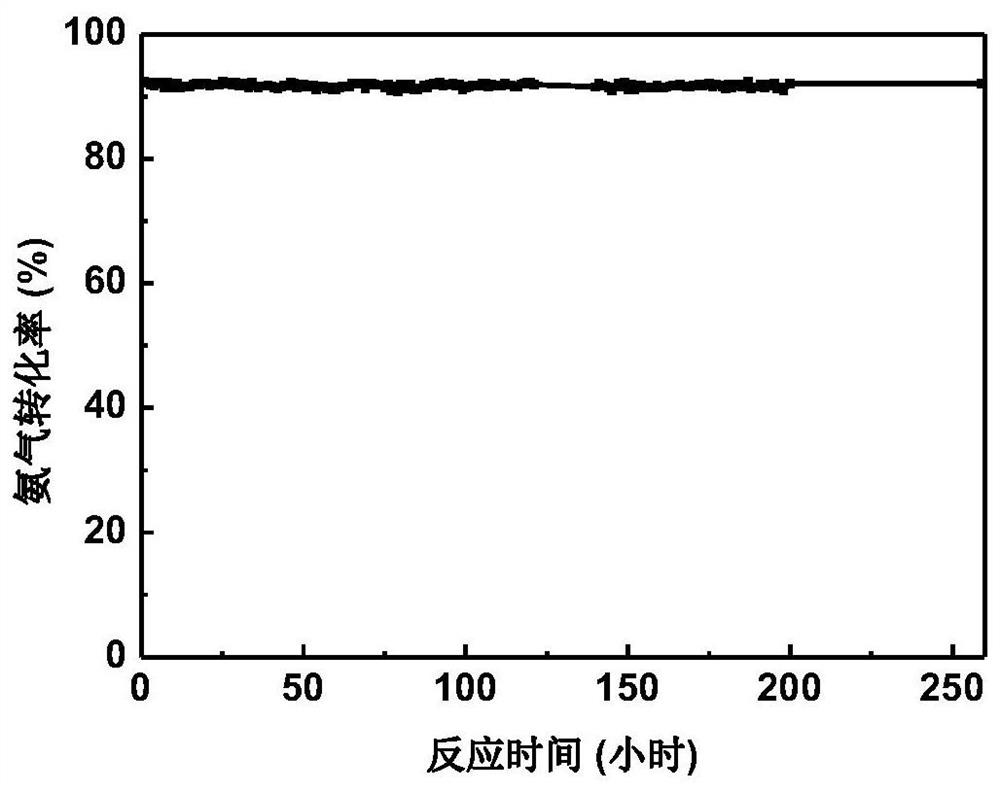
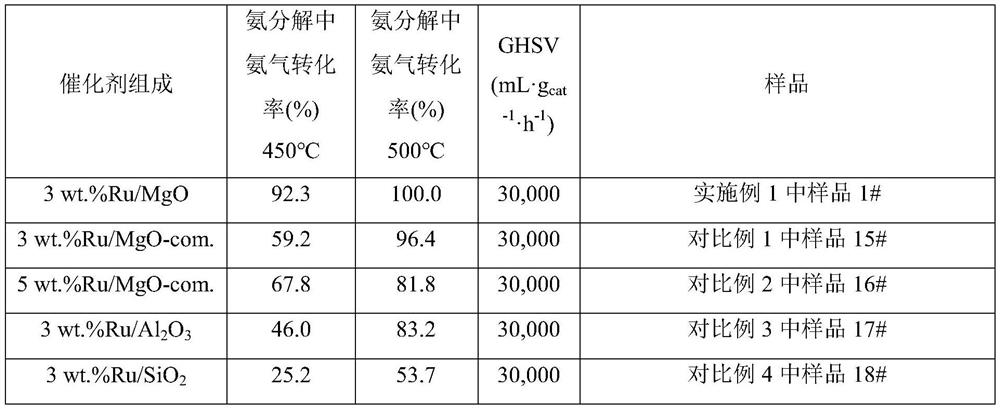
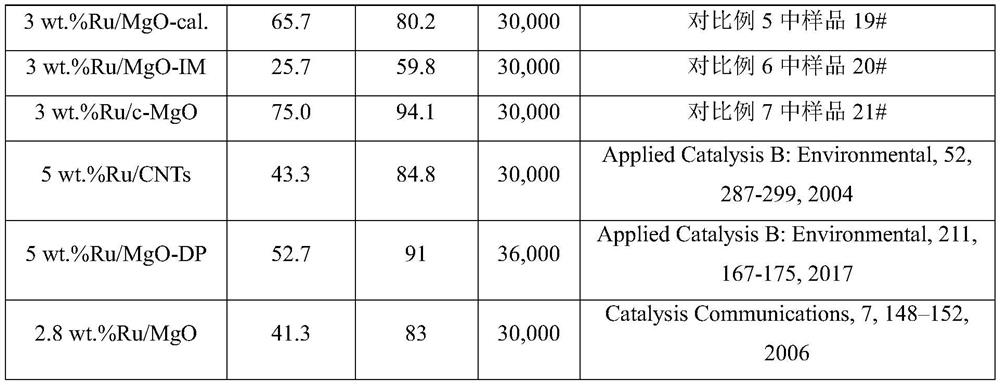
[0092] Weigh 0.08g of ruthenium chloride and dissolve it in 100mL of water, add 3.7g of magnesium oxalate to the aqueous solution of ruthenium chloride under stirring, and then add 3.6g of urea to the above suspension, then react at 80°C under stirring 8 hours. After the reaction is completed, filter and wash the product repeatedly with deionized water until the filtrate is neutral. After the product was dried, it was reduced with hydrogen at 500°C for 2 hours to obtain a magnesium oxide-supported ruthenium catalyst (3wt.%Ru / MgO) with a ruthenium loading of 3wt.%, marked as 1#.
[0093] The ammonia decomposition reaction activity of the prepared catalyst was evaluated in an ammonia decomposition device. In pure ammonia, the space velocity is 30000mL·g cat -1 h -1 1. Under the condition that the reaction pressure is normal pressure and the reaction temperature is 450° C., the conversion rate of ammonia is 92.3%. In pure ammonia, the space velocity is 20000mL·g cat -1 h ...
Embodiment 2
[0095] Weigh 0.03g of ruthenium chloride and dissolve it in 80mL of water, add 3.7g of magnesium oxalate to the aqueous solution of ruthenium chloride under stirring, stir well, then add 2.0g of urea to the above suspension, and then react at 70°C under stirring 8 hours. After the reaction is completed, filter and wash the product repeatedly with deionized water until the filtrate is neutral. After the product was dried, it was reduced with hydrogen at 500° C. for 2 hours to obtain a magnesium oxide-supported ruthenium catalyst (1 wt.% Ru / MgO) with a ruthenium loading of 1 wt.%, which was marked as 2#.
[0096] The ammonia decomposition reaction activity of the prepared catalyst was evaluated in an ammonia decomposition device. In pure ammonia, the space velocity is 10000mL·g cat -1 h -1 1. Under the condition that the reaction pressure is normal pressure and the reaction temperature is 450° C., the conversion rate of ammonia is 98.5%.
Embodiment 3
[0098] Weigh 0.08g of ruthenium chloride and dissolve it in 150mL of water, add 3.7g of magnesium oxalate into the aqueous solution of ruthenium chloride under stirring, and then add 1.0g of sodium carbonate to the above suspension, and then stir at 150°C React for 2 hours. After the reaction is completed, filter and wash the product repeatedly with deionized water until the filtrate is neutral. After the product was dried, it was reduced with hydrogen at 50° C. for 12 hours to obtain a magnesium oxide-supported ruthenium catalyst with a ruthenium loading of 3 wt.%, marked as 3#.
[0099] The ammonia decomposition reaction activity of the prepared catalyst was evaluated in an ammonia decomposition device. In pure ammonia, the space velocity is 10000mL·g cat -1 h -1 1. Under the condition that the reaction pressure is normal pressure and the reaction temperature is 450° C., the conversion rate of ammonia is 93.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com