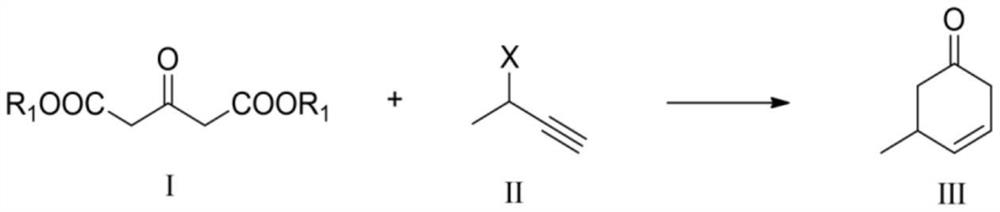
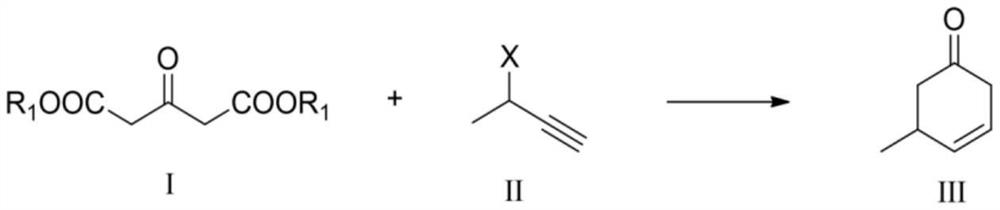
Preparation method of 5-methyl-3-cyclohexenone and application of 5-methyl-3-cyclohexenone in preparation of m-cresol
A technology of cyclohexenone and methyl, which is applied in the field of preparation, can solve problems such as harsh reaction conditions, difficult industrialization, and difficult separation, and achieve the effects of reducing processing costs, mild reaction conditions, and reducing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] (3) The preparation method of 3-butyne-2-acetate:
[0051] Dissolve 3-butyn-2-ol (5 mmol) in 10 mL of acetic anhydride, heat to reflux at 110°C for 10 h, wait for the reaction solution to cool to room temperature, rotary evaporate, and column separation to obtain 3-butyne-2 with a purity of more than 95%. - Acetate.
Embodiment 1-23
[0052] Following examples 1-23 are all used for preparing 5-methyl-3-cyclohexenone:
Embodiment 1
[0054] (1) Preparation of copper complex catalyst A
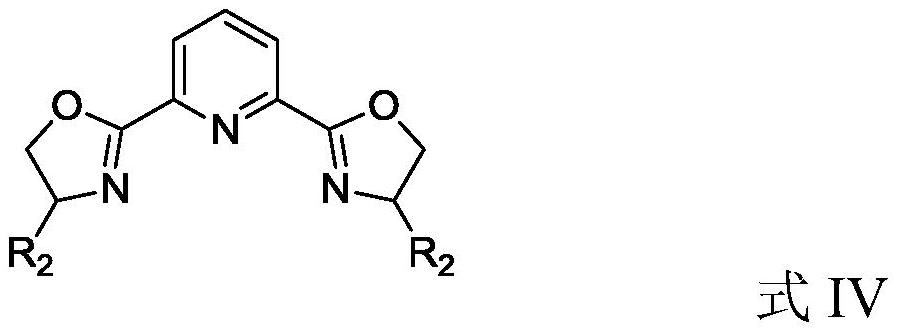
[0055] Add the metal precursor CuCl to the reaction flask 2 2H 2 O (0.02mmol) and the ligand L1 (0.025mmol) represented by the following formula, 1.0mL of anhydrous methanol was added under nitrogen protection, and stirred at room temperature for 4 hours to obtain the target product catalyst A with a molecular weight of .
[0056]
[0057] (2) dimethyl 1,3-acetonedicarboxylate (0.54mmol), 3-chloro-1-butyne (0.45mmol), and triethylamine (0.45mmol) were dissolved in 10.0mL of anhydrous methanol, and then The solution was added to the stirred catalyst A solution under the protection of nitrogen, stirred at 25° C. for 10 h (the first stage), and then stirred at 120° C. for 8 hours (the second stage). After the reaction was completed, rotary evaporation under reduced pressure and column separation gave the product 5-methyl-3-cyclohexenone with a yield of 95.2%.
[0058] 1 H NMR (CDCl 3 ,400MHz):δ6.15(m,1H),5.66(m,1H),3.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com