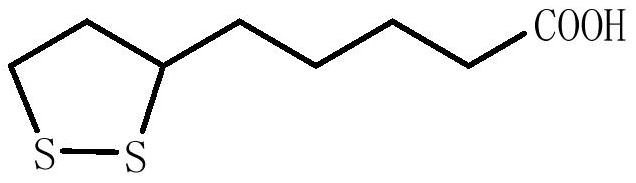
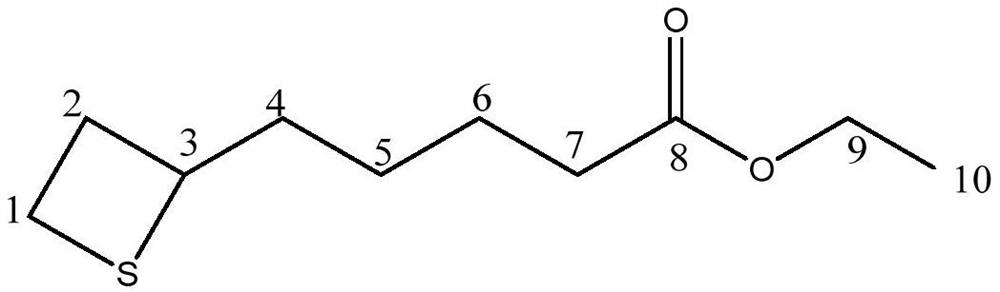
Preparation method of lipoic acid impurities
A technology of lipoic acid and impurities, applied in the field of preparation of lipoic acid impurities, to achieve a good reaction route, improve reaction conditions, and avoid the formation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The present embodiment provides a kind of preparation method of lipoic acid impurity, the preparation method of described lipoic acid impurity comprises the following steps:
[0054] (1)N 2 Under protection, ethyl 6,8-dichlorooctanoate (10g, 0.041mol), sodium sulfide pentahydrate (7.5g, 0.045mol), tetrabutylammonium bromide (0.7g, 0.002mol) and water (50mL) The mixture was stirred and reacted at 45°C for 4h; sodium sulfite (10g, 0.079mol) was added and the temperature was raised to 80°C and stirred for 0.5h;
[0055] Aftertreatment: after the reaction solution is down to room temperature, extract with 30g toluene, and the extract is concentrated to obtain 5.1g oily liquid (the purity of 5-thietanylvaleric acid ethyl ester in this oily liquid is 7.8%); Purified by column chromatography, eluting with an eluent with a volume ratio of ethyl acetate / n-heptane=1 / 10 to obtain 1.6 g of ethyl 6,8-dichlorooctanoate (purity 88.6%) and 5-sulfur A mixture of ethyl heterocyclobutan...
Embodiment 2
[0060] The present embodiment provides a kind of preparation method of lipoic acid impurity, the preparation method of described lipoic acid impurity comprises the following steps:
[0061] (1)N 2 Under protection, a mixture of ethyl 6,8-dichlorooctanoate (50g, 0.207mol), sodium sulfide pentahydrate (37.5g, 0.223mol), tetrabutylammonium bromide (2g, 0.006mol) and water (50mL) The reaction was stirred at room temperature for 24h. Sodium sulfite (50g, 0.397mol) was added and the temperature was raised to 80°C and the reaction was stirred for 1h;
[0062] Post-treatment: After the reaction liquid was lowered to room temperature, it was extracted with 100 g of toluene, and concentrated to obtain 16 g of oily liquid (the purity of ethyl 5-thietanylvalerate in the oily liquid was 7.9%);
[0063] (2) Sulfur (2.7g, 0.084mol), tetrabutylammonium bromide (1g, 0.003mol) and water (50mL) were added to the oily liquid obtained in step (1), heated to 80°C under stirring, and A solution o...
Embodiment 3
[0067] The present embodiment provides a kind of preparation method of lipoic acid impurity, the preparation method of described lipoic acid impurity comprises the following steps:
[0068] (1)N 2 Under protection, ethyl 6,8-dichlorooctanoate (50g, 0.207mol), sodium sulfide pentahydrate (37.5g, 0.223mol), tetrabutylammonium bromide (2g, 0.006mol) and water (50mL) The mixture was stirred at 25°C for 24h. Sodium sulfite (50g, 0.397mol) was added and the temperature was raised to 80°C and the reaction was stirred for 1h;
[0069] Post-processing: After the reaction liquid was lowered to room temperature, it was extracted with 30 g of toluene to obtain a toluene liquid;
[0070] (2) Sulfur (2.7g, 0.084mol), tetrabutylammonium bromide (1g, 0.003mol) and water (50mL) were added to the toluene liquid obtained in step (1), heated to 80°C under stirring, and A solution of sodium sulfide pentahydrate (12 g, 0.071 mol) in water (30 mL) was added dropwise at temperature. After droppin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com