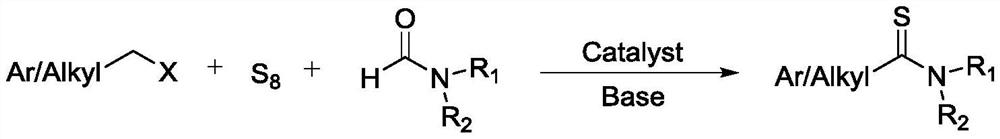
Synthetic method of thioamide compound
A technology of thioamide and synthetic method, which is applied in the synthesis of organic compounds and the field of synthesis of pharmaceutical intermediates containing thioamide structural units, can solve the problems of low atom utilization, poor step economy, unfriendly environment, etc., and achieve improvement Effects of atom economy, short reaction time, and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, a kind of synthetic method of thioamide compound, take iodopropane as raw material:
[0041] Under nitrogen protection, iodopropane (0.67g, 3.9mmol), sublimed sulfur (0.25g, 7.8mmol), sodium hydroxide (0.47g, 11.7mmol) were added to 5mL N,N-dimethylformamide, and then Cuprous chloride (0.04 g, 0.39 mmol) and ethylenediaminetetraacetic acid (0.23 g, 0.78 mmol) were added, and the reaction was stirred at room temperature for 12 hours.
[0042] After the reaction was completed, 5 mL of deionized water was added to the resulting reaction liquid to quench the reaction liquid, followed by extraction with ethyl acetate (5 mL×3) to obtain an aqueous phase and an organic phase, respectively, and then saturated brine (5 mL×3) The organic phase was washed and dried with anhydrous sodium sulfate, filtered (to remove sodium sulfate), concentrated by rotary evaporation (to remove ethyl acetate), purified by silica gel (100-200 mesh) column chromatography, and purified w...
Embodiment 2
[0043] Embodiment 2, a kind of synthetic method of thioamide compound, take chlorooctane as raw material:
[0044] Under nitrogen protection, chlorooctane (0.58g, 3.9mmol), sublimed sulfur (0.25g, 7.8mmol), sodium hydroxide (0.47g, 11.7mmol) were added to 5mL N,N-dimethylformamide, Then cuprous chloride (0.04g, 0.39mmol) and ethylenediaminetetraacetic acid (0.23g, 0.78mmol) were added, and the reaction was stirred for 10 hours at a reaction temperature of 40°C.
[0045]After the reaction was completed, 5 mL of deionized water was added to the resulting reaction liquid to quench the reaction liquid, followed by extraction with ethyl acetate (5 mL×3) to obtain an aqueous phase and an organic phase, respectively, and then saturated brine (5 mL×3) The organic phase was washed, dried with anhydrous sodium sulfate, filtered, concentrated by rotary evaporation, purified by silica gel (100-200 mesh) column chromatography, and eluted with a mixture of ethyl acetate / petroleum ether (1:8...
Embodiment 3
[0046] Embodiment 3, a kind of synthetic method of thioamide compound, take bromomethylcyclohexane as raw material:
[0047] Under nitrogen protection, bromomethylcyclohexane (0.69g, 3.9mmol), sublimed sulfur (0.25g, 7.8mmol), sodium hydroxide (0.47g, 11.7mmol) were added to 5mL N,N-dimethylformaldehyde Add cuprous chloride (0.04g, 0.39mmol) and ethylenediaminetetraacetic acid (0.23g, 0.78mmol) to the amide, and stir the reaction for 12 hours at a reaction temperature of 60°C.
[0048] After the reaction was completed, 5 mL of deionized water was added to the resulting reaction liquid to quench the reaction liquid, followed by extraction with ethyl acetate (5 mL×3) to obtain an aqueous phase and an organic phase, respectively, and then saturated brine (5 mL×3) The organic phase was washed, dried with anhydrous sodium sulfate, filtered, concentrated by rotary evaporation, purified by silica gel (100-200 mesh) column chromatography, and eluted with a mixture of ethyl acetate / pet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com