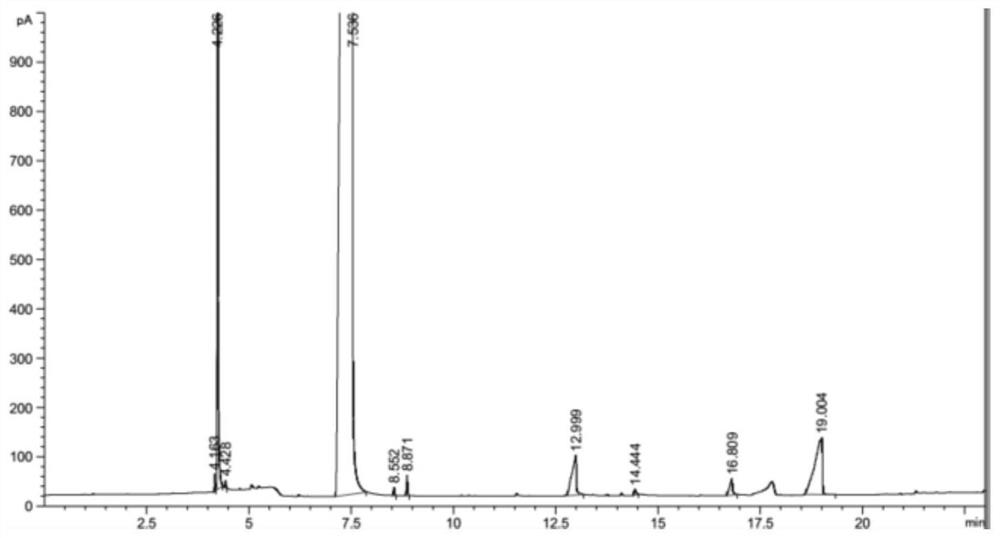
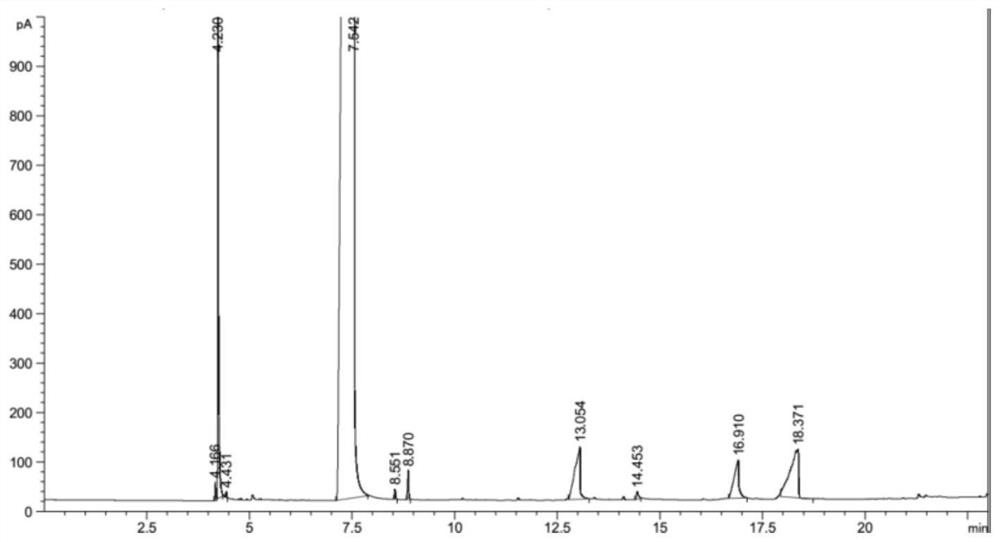
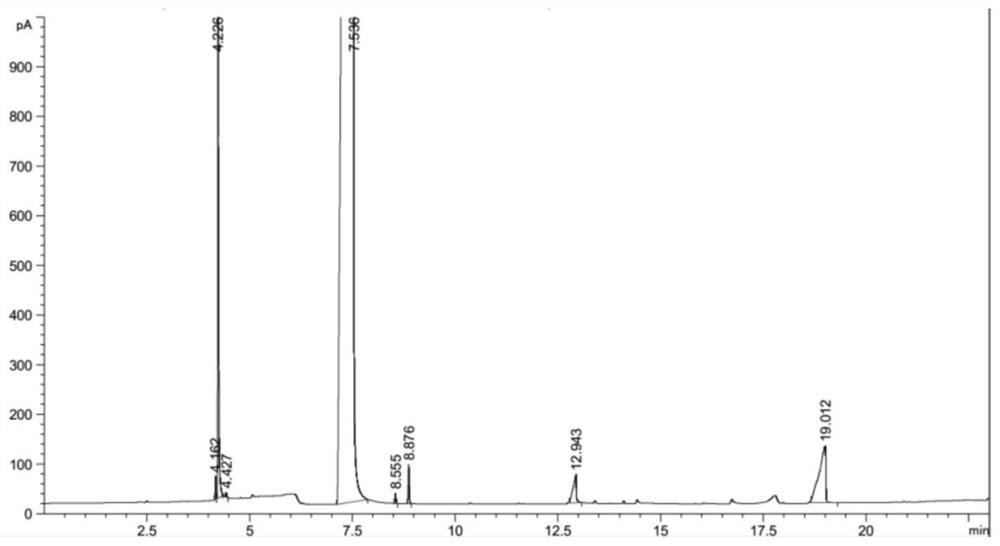
Method for producing methyl thioglycolate based on sodium hydrosulfide method
A technology of methyl thioglycolic acid and thioglycolic acid, applied in the field of producing methyl thioglycolic acid, can solve the problems of long reaction time, high cost, lower yield of finished products, etc. The effect of improving the quality and enhancing the activity of molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) 1000mL of thioglycolic acid acidification solution synthesized by the sodium hydrosulfide method is added in portions to a distillation flask, concentrated by decompression distillation and dehydration, and centrifuged to separate NaCl solid to obtain 200 g of distillation concentrated solution; the concentration of thioglycolic acid is 50wt.%.
[0043] (2) Slowly add CaCl to the distillation concentrate 2 100g, CaCl 2 Form the molten salt hydrate with the water in the distilled concentrate, at this moment, there will be solid NaCl to separate out constantly, after separating out the NaCl solid, merge with the NaCl solid of the centrifugal separation of step (1) gained to form solid salt; Then use 2 times the mass of methanol to wash the solid salt twice, the methanol deesterification reaction process after washing the solid salt, and the washed solid salt is used as a by-product after drying.
[0044](3) Add 0.3 g of p-toluenesulfonic acid and 103 g of methanol ...
Embodiment 2
[0051] (1) 1000mL of thioglycolic acid acidification solution synthesized by the sodium hydrosulfide method is added in portions to a distillation flask, concentrated by decompression distillation and dehydration, and centrifuged to separate the NaCl solid to obtain 150 g of distillation concentrated solution; the concentration of thioglycolic acid is 65wt.%.
[0052] (2) Slowly add the distillation concentrate of step (1) to the lower aqueous phase of Example 1, where the CaCl 2 Hydrate is molten salt hydrate. At this time, there will be solid NaCl precipitated continuously. After the separated NaCl solid is separated, it will be combined with the centrifuged NaCl solid obtained in step (1) to form a solid salt; then use 2 times the mass of methanol The solid salt is washed 3 times, and the methanol deesterification reaction process after washing the solid salt is used as a by-product after the washed solid salt is dried.
[0053] At the same time, add a small amount of CaCl...
Embodiment 3
[0062] (1) 700mL of thioglycolic acid acidification solution synthesized by the sodium hydrosulfide method is added in portions to a distillation flask, concentrated by decompression distillation and dehydration, and centrifuged to separate NaCl solid to obtain distillation concentrated solution 100g; the concentration of thioglycolic acid is 69.5 wt.%.
[0063] (2) Slowly add the distillation concentrated solution of step (1) to the lower layer aqueous phase concentrated solution of Example 2, wherein the CaCl 2 Hydrate is molten salt hydrate. At this time, there will be solid NaCl precipitated continuously. After the separated NaCl solid is separated, it will be combined with the centrifuged NaCl solid obtained in step (1) to form a solid salt; then use 2 times the mass of methanol The solid salt is washed twice, and the methanol deesterification reaction process after washing the solid salt is used as a by-product after the washed solid salt is dried.
[0064] At the same ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com