Medicine-loaded medical instrument and preparation method thereof
A medical device and drug-loading technology, which is applied in the field of drug-loaded medical devices and its preparation, can solve problems such as difficult preparation of macrolide crystals, poor drug transfer effect, weak interaction force, etc., to improve adhesion performance , extend the concentration, improve the effect of transfer speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention also relates to a method for preparing a drug-loaded medical device, characterized in that the method comprises:
[0056] 1) preparing macrolide drug crystal powder, dispersing the powder in a solvent, adding rigid microspheres for shaking, filtering and removing the rigid microspheres to obtain a suspension;
[0057] 2) Take a medical device body with a solid surface, apply the suspension on the solid surface, and dry to obtain a medical device intermediate;
[0058] 3) Applying the supersaturated solution of the macrolide drug on the surface of the medical device intermediate and drying.
[0059] In some embodiments, the suspension contains crystalline microparticles of macrolide drugs with a particle size of 100 nm to 1000 nm.
[0060] In some embodiments, the particle size of the crystalline microparticles can also be 200nm, 300nm, 400nm, 500nm, 600nm, 700nm, 800nm, 900nm. Further 200nm~500nm.
[0061] In some embodiments, the purity of the ...
Embodiment 1
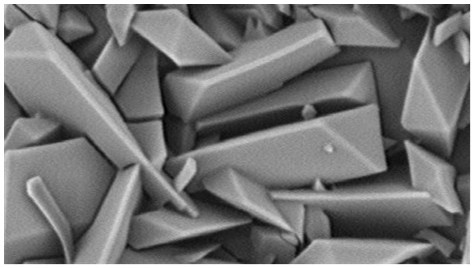
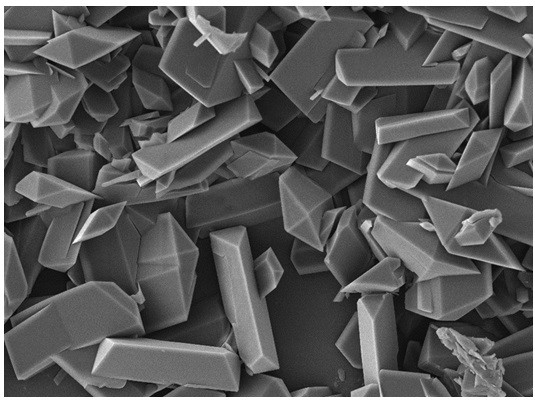
[0075] Take 1 g of rapamycin raw material and disperse it in 50 mL of n-heptane, add a stirrer, stir overnight at room temperature and avoid light, stirring speed is 300 rpm, then filter to obtain filter cake, and vacuum dry to obtain crystalline rapamycin , Use infrared spectroscopy or powder X-ray diffraction to measure the crystallinity, and determine that the crystallinity is above 90%.
[0076] Take 7 mg of the above crystalline rapamycin in a brown bottle, add 10 mL of n-heptane to disperse the rapamycin, then add 2.5 g of zirconia beads (Φ0.2-0.3 mm), seal it, and fix it in a vortex to mix On the instrument, shake for 10 minutes, and the shaking speed is 2500rpm. After oscillating, put it into a water-bath ultrasonic sonicator for 3 min, and then filter it with a 40 μm nylon filter to remove the zirconia beads and obtain the filtrate. Use a microsampler to draw 20 μL and evenly coat the surface of the balloon, and let it dry naturally for 2 hours in the dark.
[0077]...
Embodiment 2
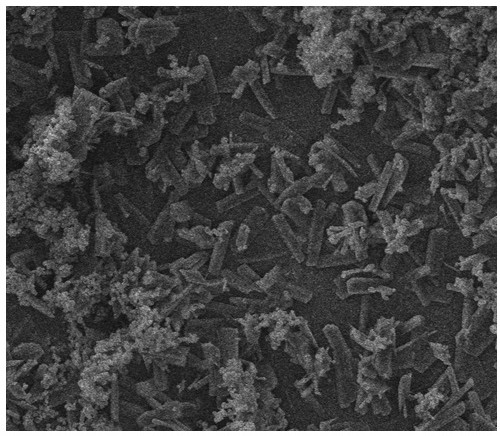
[0080] Take 1 g of everolimus raw material and disperse it in 50 mL of n-heptane, add a stirrer, stir overnight at room temperature and avoid light at a stirring speed of 300 rpm, then filter to obtain a filter cake, and vacuum-dry to obtain crystalline everolimus , Use infrared spectroscopy or powder X-ray diffraction to measure the crystallinity, and determine that the crystallinity is above 90%.
[0081] Take 7 mg of the above crystalline everolimus in a brown bottle, add 10 mL of n-heptane to disperse the everolimus, then add 2.5 g of zirconia beads (Φ0.2-0.3 mm), seal it, and fix it in a vortex to mix On the instrument, shake for 10 minutes, and the shaking speed is 2500rpm. After oscillating, put it into a water-bath ultrasonic bath for 3 min, and then filter it with a 40 μm nylon filter to remove the zirconia beads and obtain the filtrate. Use a microsampler to draw 20 μL and evenly coat it on the surface of the balloon, and let it dry naturally for 2 hours in the dark...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com