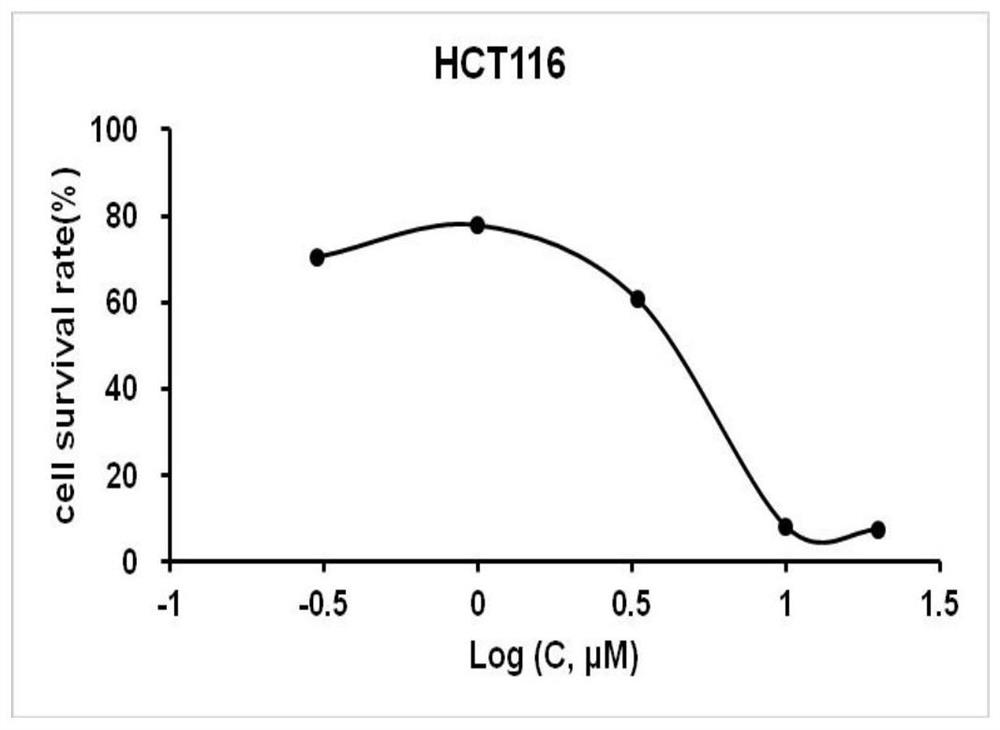
Amino acid derivative bithiazole-tryptamine anticancer compound and application
An amino acid, bithiazole technology, applied in organic chemistry, anti-tumor drugs, drug combinations, etc., can solve the problems of destruction of tissues, organ structure and function, necrosis and bleeding complicated by infection, etc., to achieve good anti-cancer effect, broad market prospects and Scientific value, simple route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
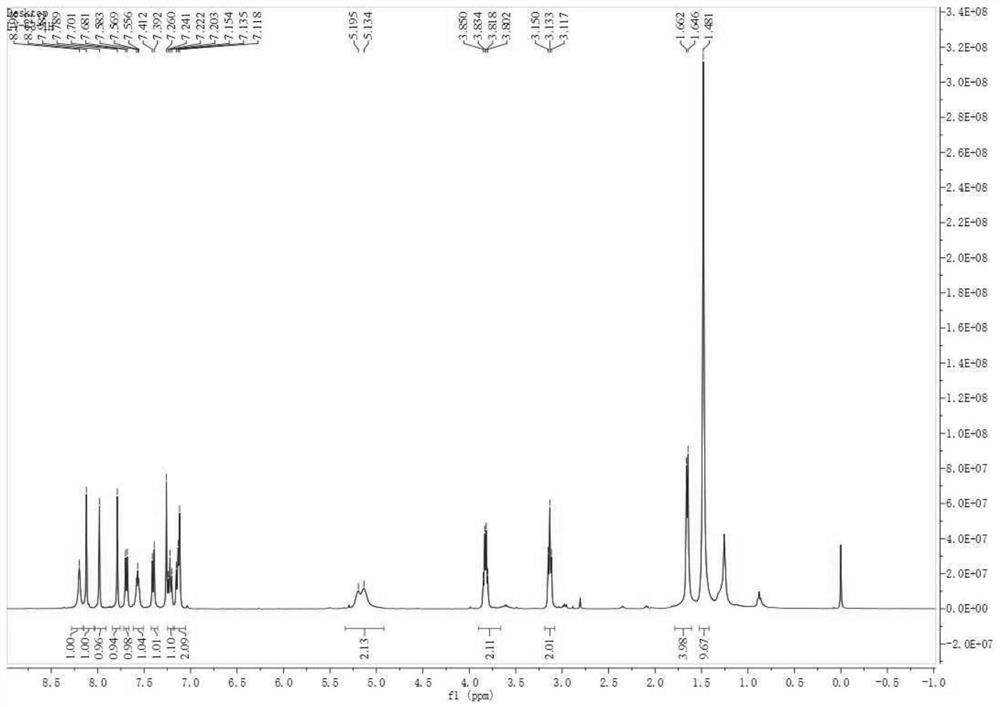
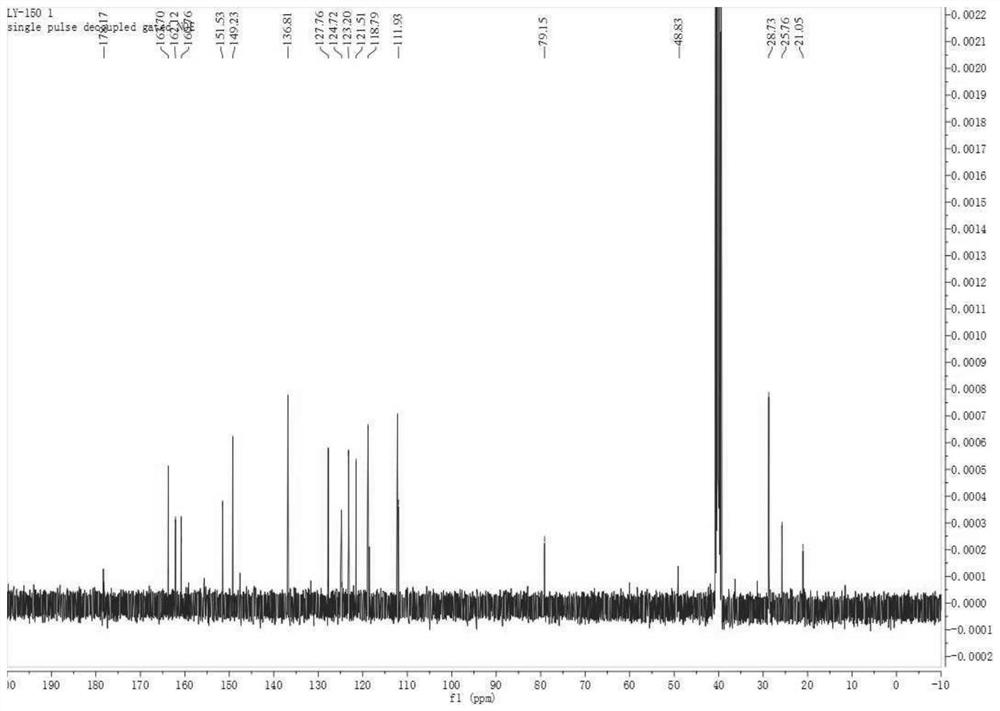
[0030] Compound (3) of Example 1
[0031] Step: 4mmol Boc-L-Ala-COOH and 4mmol pentafluorophenyl diphenyl phosphate were dissolved in 80ml dichloromethane, after stirring at room temperature for 10min, 10mmol triethylamine, 8mmol triphenylphosphine and 1mmol disulfide azide Compound 2 was added to the reaction system in sequence, and heated to reflux until the reaction was monitored by TLC; after the reaction system was cooled to room temperature, 10 mmol of bromotrichloromethane and 12 mmol of 1,8-diazabicyclodeca were slowly added to the system One-carb-7-ene (DBU), continue to react at room temperature for 1 hour, add saturated ammonium chloride solution to quench the reaction, extract with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, and remove the solvent by rotary evaporation to obtain a brown viscous The thick liquid was separated by column chromatography to obtain the target thiazole compound 3 with a yield of 78%. [α] D 20 -36.4(c...
Embodiment 2
[0032] Example 2 compound (5)
[0033] Step: 0.5mmol of compound 3 was dissolved in 10ml of a mixture of methanol and tetrahydrofuran (volume ratio 1:1), under ice water conditions, 5ml of 0.5N sodium hydroxide solution was added to the system, and TLC was monitored until the reaction was complete. Under water conditions, carefully add 0.5N hydrochloric acid solution to the system to adjust the pH value to 2-3, extract three times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, spin dry, and directly use in the next reaction. Dissolve the prepared carboxylic acid and 0.5 mmol of pentafluorophenyl diphenyl phosphate in 10 ml of dichloromethane, stir at room temperature for 10 min, then dissolve 1.5 mmol of triethylamine, 1.5 mmol of triphenylphosphine and 0.125 mmol of disulfide azide Compound 2 was added to the reaction system in sequence, heated to reflux until the reaction was monitored by TLC; after the reaction system was cooled to room t...
Embodiment 3
[0034] Example 3 compound (7)
[0035] Steps: Dissolve 0.5mmol of compound 5 in 10ml of a mixture of methanol and tetrahydrofuran (1:1), add 5ml of 0.5N sodium hydroxide solution to the system under ice water conditions, and monitor by TLC until the reaction is complete. Next, carefully add 0.5N hydrochloric acid solution to the system to adjust the pH value to 2-3, extract three times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and spin dry, then directly use in the next reaction. Dissolve the prepared carboxylic acid and 0.5 mmol of pentafluorophenyl diphenyl phosphate in 10 ml of dichloromethane, stir at room temperature for 10 min, then dissolve 1.5 mmol of triethylamine, 1.5 mmol of triphenylphosphine and 0.125 mmol of disulfide azide Compound 2 was added to the reaction system in sequence, heated to reflux until the reaction was monitored by TLC; after the reaction system was cooled to room temperature, 2.0 mmol of bromotrichloromet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com