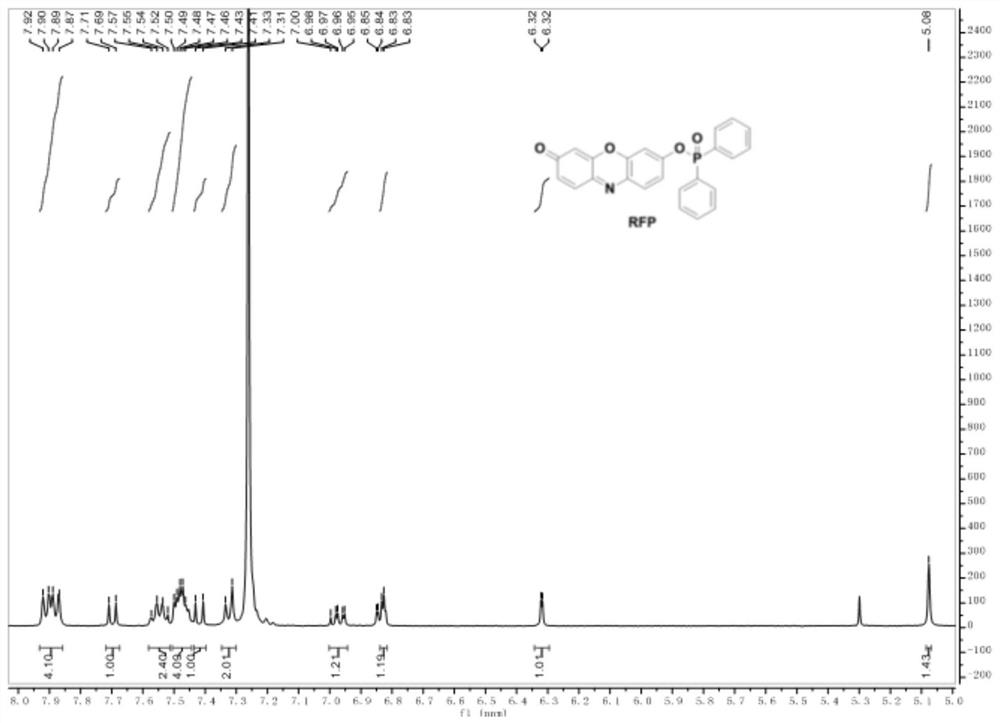
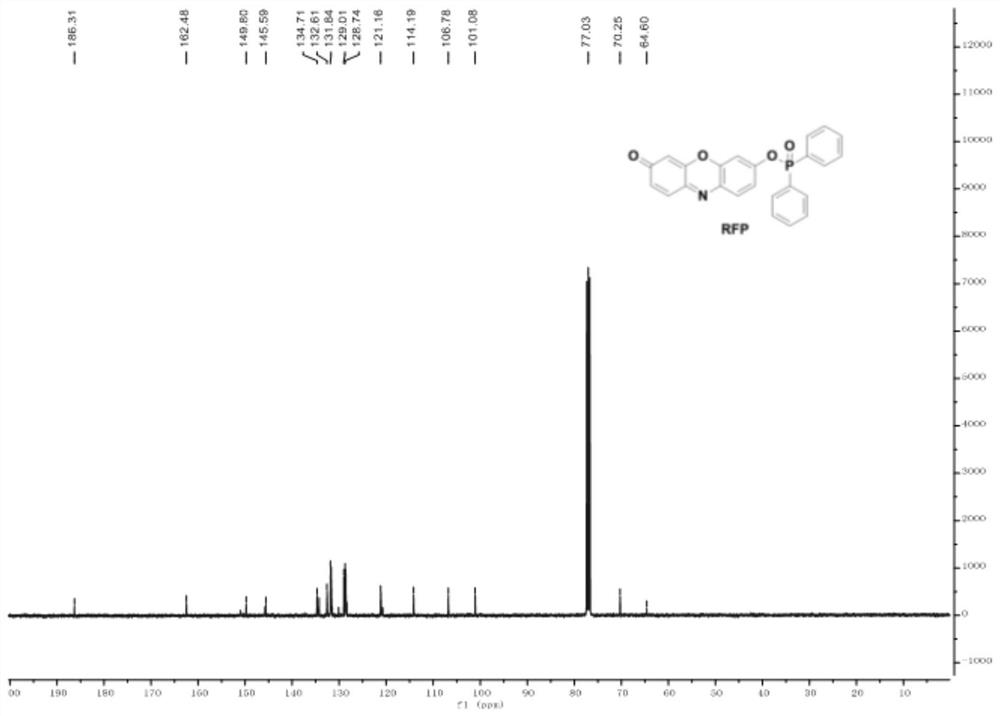
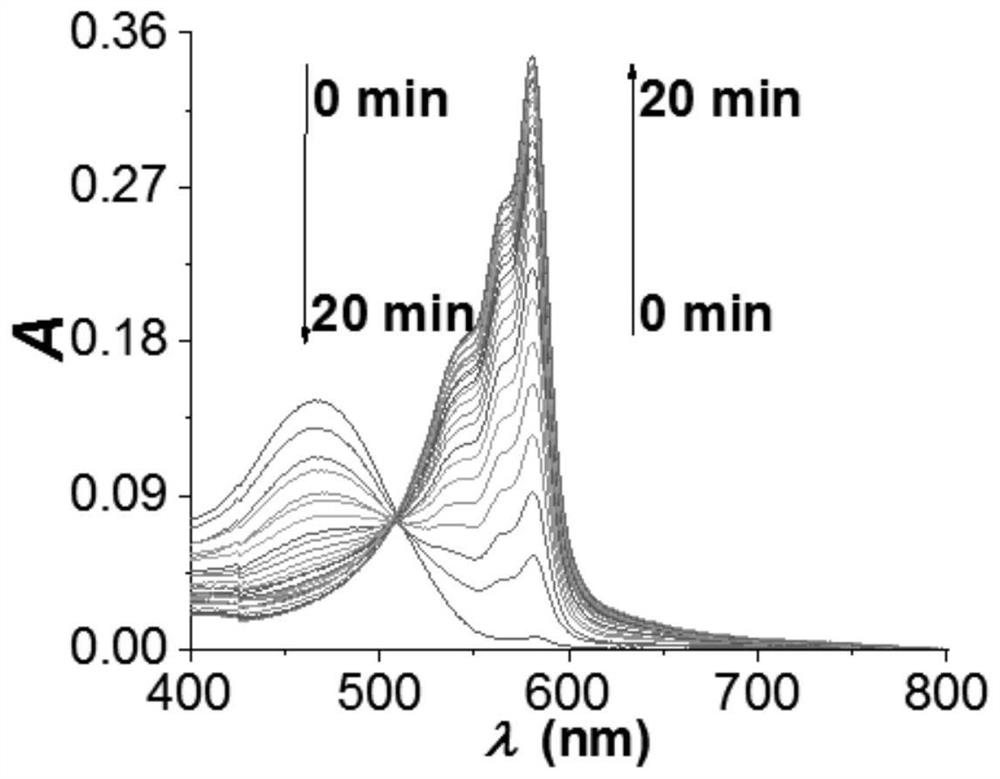
Fluorescent probe based on resorufin dye specific response ONOO<->, and preparation method and application thereof
A fluorescent probe and resorufin technology, applied in the field of fluorescent probes, can solve the problems of lack of imaging applications, short fluorescence emission wavelength, low selectivity, etc., and achieve the effects of obvious detection signal, low cytotoxicity, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation steps of the probe are as follows:
[0041] (1) Preparation of Compound 1
[0042] ;
[0043] N 2 Under the protection of an ice-water bath, dissolve p-hydroxybenzyl alcohol in a mixed solvent, then add diphenylphosphinyl chloride, react at room temperature for 1-2 hours, quench with ice water, extract with dichloromethane, and separate by column chromatography to obtain compound 1;
[0044] (2) Preparation of Compound 2
[0045] ;
[0046] Dissolve the compound 1 prepared in step (1) in dichloromethane, then add carbon tetrabromide and triphenylphosphine into the above system according to the ratio of equivalent 1:1~2, stir and react at room temperature for 1~2 hours, and then The solvent was removed under reduced pressure, and the compound 2 was obtained by column chromatography;
[0047] (3) Preparation of probe RFP
[0048] ;
[0049]First dissolve resorufin and potassium carbonate in N,N-dimethylformamide at a ratio of 1:2 equivalent, a...
Embodiment 2
[0052] The preparation steps of the probe are as follows:
[0053] (1) Preparation of Compound 1
[0054] N 2 Under the protection of ice-water bath, triethylamine was added into the tetrahydrofuran solvent and mixed to obtain a mixed solvent, and p-hydroxybenzoic acid oxime ester and diphenylphosphinoyl chloride were dissolved in the mixed solvent according to the ratio of equivalent of 1:1.5, and reacted at room temperature for 2 hours, quenched with ice water, extracted with dichloromethane, and separated by column chromatography to obtain compound 1;
[0055] (2) Preparation of Compound 2
[0056] Dissolve compound 1 in dichloromethane, add carbon tetrabromide and triphenylphosphine in an equivalent ratio of 1:1.5, stir at 25°C for 1-1.5h, then remove the solvent under reduced pressure, and separate by column chromatography to obtain compound 2;
[0057] (3) Preparation of compound 3
[0058] First dissolve resorufin and potassium carbonate in N,N-dimethylformamide in...
Embodiment 3
[0060] The preparation steps of the probe are as follows:
[0061] (1) Preparation of Compound 1
[0062] N 2 Under the protective ice-water bath, triethylamine was added into the tetrahydrofuran solvent and mixed to obtain a mixed solvent, and p-hydroxybenzyl alcohol and diphenylphosphinoyl chloride were dissolved in the mixed solvent in a ratio of 1:2 in equivalent, and reacted at room temperature for 2 hours, and the Quenched with water, extracted with dichloromethane, and separated by column chromatography to obtain compound 1;
[0063] (2) Preparation of Compound 2
[0064] Dissolve compound 1 in dichloromethane, add carbon tetrabromide and triphenylphosphine in an equivalent ratio of 1:2, stir at 25°C for 1-1.5h, then remove the solvent under reduced pressure, and separate by column chromatography to obtain compound 2;
[0065] (3) Preparation of compound 3
[0066] First dissolve resorufin and potassium carbonate in N,N-dimethylformamide at a ratio of 1:2 equivalen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com