Preparation method of uridine diphosphate glucuronic acid
A technology of uridine diphosphate glucose and aldehyde acid, which is applied in the field of enzyme catalysis, can solve the problems of low synthesis efficiency, high cost, and high cost of raw materials, and achieve the effects of efficient synthesis, cost saving, and controllable reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of UDP-glucose
[0034] Weigh 50g of anion exchange resin into a 250mL beaker, add 50mL of 10% isopropanol to soak for 20min, filter with suction, rinse with 10% isopropanol, rinse with deionized water, filter with suction, rinse with 0.5M HCl, filter with suction, Resuspend in 45mL 0.5M HCl, soak for 2h, filter with suction, rinse with deionized water, and stir in 45mL CoCl2 aqueous solution overnight. Wash with 50mM pH7.5 phosphate buffer for later use. Prepare 20mM Tris buffer, adjust the pH to 7.0, mix the volume of enzyme solution and buffer solution at a ratio of 1:4, take 194 mg of purified protein GlmU enzyme and 3 mg of PPA enzyme and place them in a triangular flask, and add 21.5 mg of the treated resin respectively. g, 0.15g, put into a shaker, the rotation speed is 120rpm, the temperature is 25°C, and the time is 1h. Suction filter, rinse with 50mM pH7.5 phosphate buffer, and store at 4°C for later use.
[0035] Weigh 2.16g o...
Embodiment 2
[0037] Example 2: Single enzyme catalyzes the oxidation of UDP-glucose to generate UDP-glucuronic acid
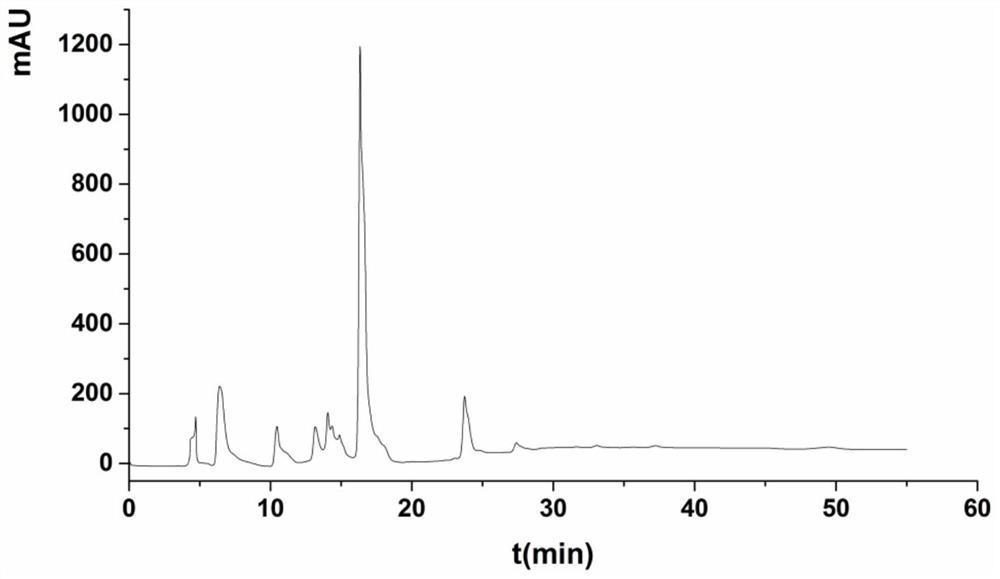
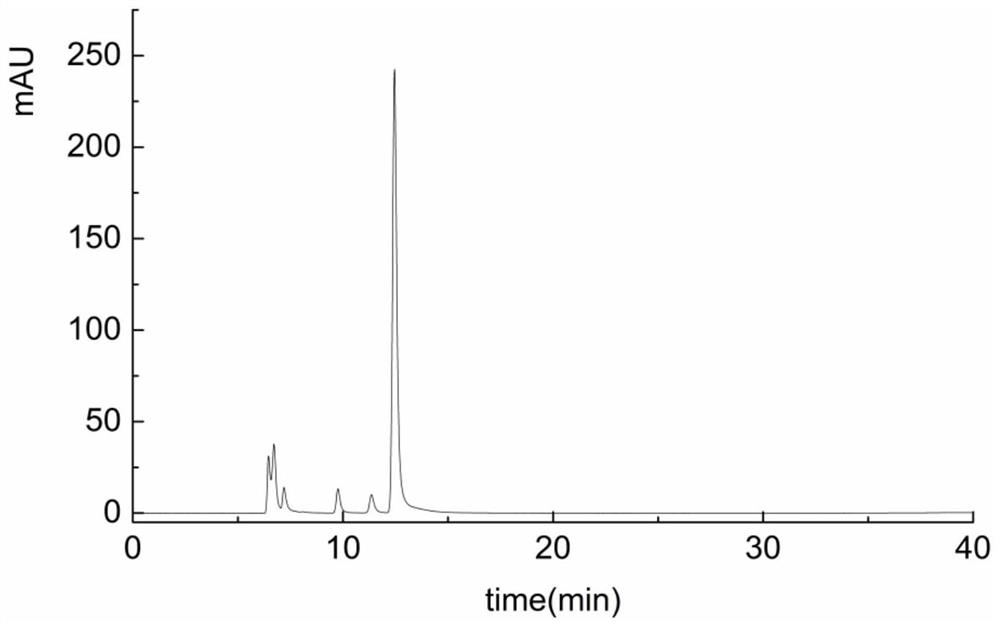
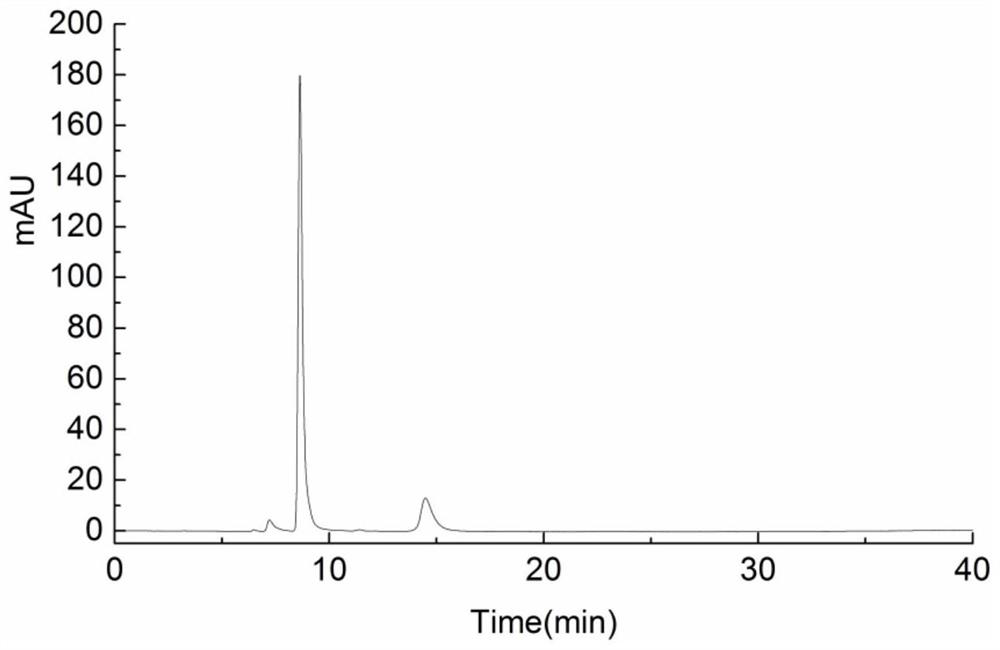
[0038] Mix 2.0mL 10mM UDPG, 2.5mL 10mM NAD, 100μL 1M Tris, 10μL β-mercaptoethanol, 20μL 1M MgCl2, 3.37mL deionized water, then add 2.5mL 1.0mg / mL UGD enzyme solution, at the optimum temperature React overnight at 25°C; take 100 μL of the reaction solution to pass through an aqueous filter membrane with a pore size of 0.22 μm, and perform HPLC analysis (such as Figure 6 shown), and the reaction substrates UDP-glucose, NAD + and product NADH, UDP-glucuronic acid standards for HPLC analysis (such as figure 2 , 3 , 4, 5 shown), Figure 6 It is shown that there is a preliminary conclusion that there are new substances NADH and UDP-glucuronic acid formed, and the calculated amount of UDP-glucuronic acid is about 0.51mg / mL.
Embodiment 3
[0039] Example 3: Dual-enzyme coupling catalyzes the oxidation of UDP-glucose to generate UDP-glucuronic acid
[0040] Mix 2.4mL 10mM UDPG, 1.5mL 10mM NAD, 100μL 1M Tris, 10μL β-mercaptoethanol, 20μL 1M MgCl2, 1mL 1M sodium pyruvate, 1.97mL deionized water, and then add 2.5mL 1.0mg / mL UGD enzyme solution, 0.5mL 2.7mg / mL LDH enzyme solution, and react overnight at the optimum temperature of 25°C; take 100μL of the reaction solution to pass through an aqueous filter membrane with a pore size of 0.22μm for HPLC analysis, and UDP-glucosaldehyde can be detected acid generation, the yield was about 1.01mg / mL, and the yield was nearly doubled (such as Figure 7 shown).
[0041] The UDP-glucuronic acid standard product and the reaction product UDP-glucuronic acid were detected by mass spectrometry, and the LC-MS analysis was carried out by WATERSMALDI SYNAPT MS, as shown in Figure 8 , 9 As shown, the test result of the sample is consistent with the test result of the UDP-glucuronic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com