Novel coronavirus nucleic acid chromatography detection kit and application
A detection kit and coronavirus technology, which is applied in the determination/inspection of microorganisms, resistance to vector-borne diseases, DNA/RNA fragments, etc., which can solve the problems of the possibility of aerosol pollution, difficult DNA degradation, and low specificity , to achieve the effects of low equipment requirements, easy degradation, and low possibility of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of nucleic acid detection test strips
[0053] The main raw materials needed in the preparation of nucleic acid detection test strips: nitrocellulose membrane (NC membrane), sample pad, absorbent paper, PVC bottom plate, etc.
[0054] 1. Spray film:
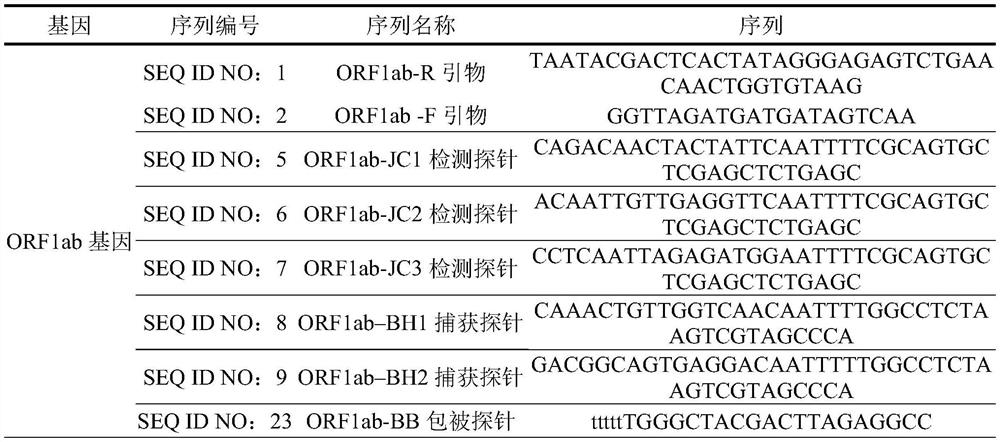
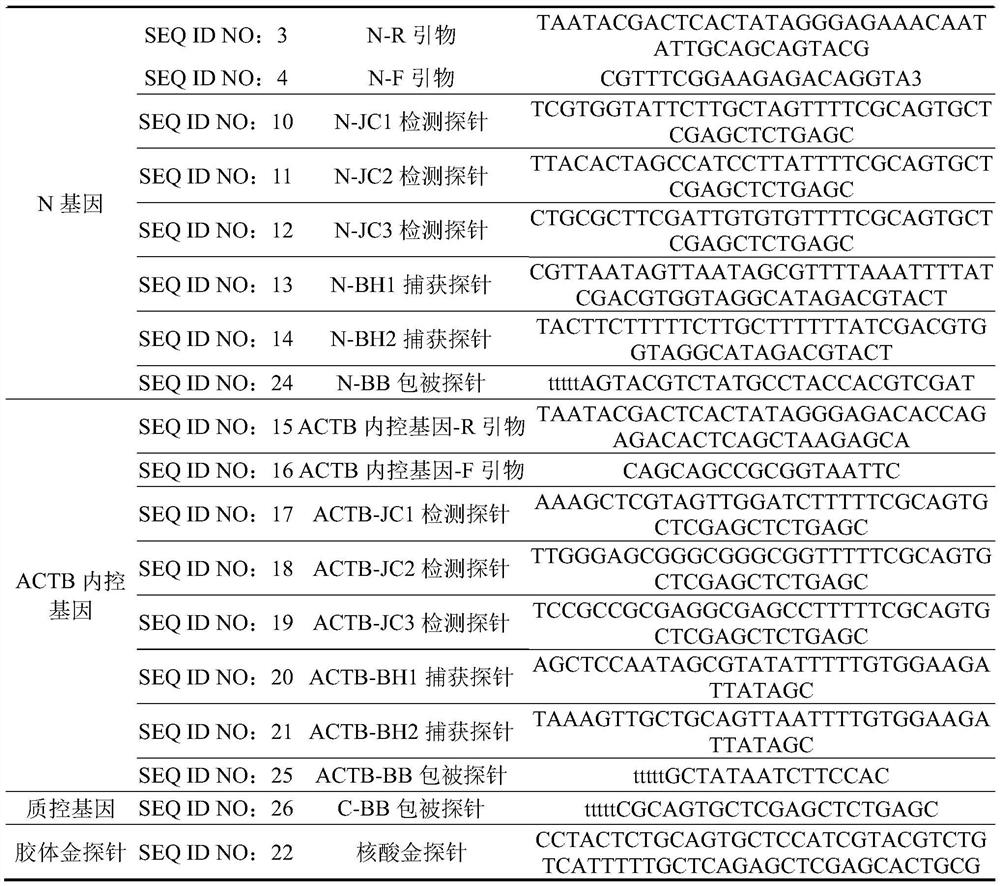
[0055] Detection line N-T line: N gene coated probe sequence modified by biotinylation at the 5' end, (20μM), spray volume: 2-3μL / cm;
[0056] Detection line ORF1ab-T line: 5' end biotinylated modified ORF1ab gene coating probe sequence, (20μM), spray volume: 2-3μL / cm;
[0057] Internal reference of detection line-T line: ACTB gene-coated probe sequence modified by biotinylation at the 5' end, (20 μM), spray volume: 2-3 μL / cm;
[0058] Quality control line (line C): biotinylated modification at the 5' end, capable of capturing nano-gold probes (20 μM), spray volume: 2-3 μL / cm.
[0059] After spraying the film, put it in a clean incubator at 37°C to dry for 2 hours, and store it in a dry environment for later...
Embodiment 2
[0063] Sensitivity test comparison test test
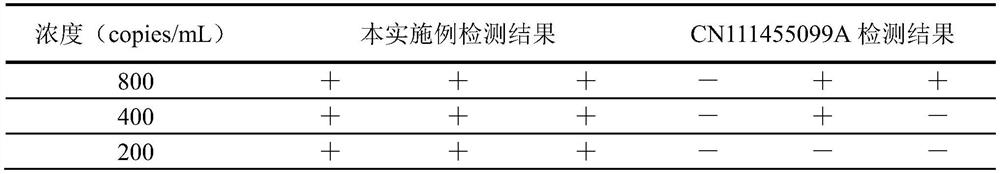
[0064] The colloidal gold prepared by this development method and the method published in Chinese patent application CN111455099A was used for a sensitivity comparison test. Select a known concentration of the pseudovirus containing the 2019-nCoV target gene, perform a 10-fold concentration gradient dilution, repeat each gradient 3 times, and use the lowest dilution concentration with a 100% positive detection rate as the estimated detection limit, the estimated detection limit After determination, the 2019-nCoV pseudovirus was diluted to near the estimated detection limit concentration, and tested with a kit, and each concentration was tested 20 times to further accurately determine the lowest detection limit concentration (select the dilution with a positive rate of more than 95%) degree as the detection limit sensitivity of this kit).
[0065] Table 2 Determination of the detection limit of 2019-nCoV
[0066]
[0067]
...
Embodiment 3
[0070] specificity verification
[0071] Other pathogens that are similar in species to 2019 novel coronavirus or cause similar symptoms, such as seasonal influenza A H1N1 virus, novel influenza A H1N1(2009) virus, influenza A H3N2, H7N9, influenza B Yamagata, influenza B Victoria, Respiratory Syncytial Virus Type A, Respiratory Syncytial Virus Type B, Parainfluenza Type I, Parainfluenza Type II, Parainfluenza Type III, Rhinovirus Groups A, B, C, Adenovirus Type 1, 7, Enterovirus A , B, C, D types, rotavirus group A, norovirus type GI, Mycoplasma pneumoniae, Chlamydia pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, coronavirus (HKU1, OC43, NL63, 229E ), a MERS coronavirus pseudovirus, a cross-reaction test was carried out to verify the specificity of the kit’s detection. The results showed that there was no cross-reaction between the kit and other microorganisms, reflecting the strong specificity of the kit’s detection of pathogens.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com