Method for producing tagatose by immobilizing multiple enzymes in artificial oil bodies
A technology of immobilized enzymes and tagatose, applied in the field of genetic engineering, can solve the problems of reducing production costs, high production costs, high production costs, etc., and achieve the effects of reducing production costs, simplifying the preparation process, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Construction of expression vector and fusion protein expression
[0038] 1. Construction of expression vector
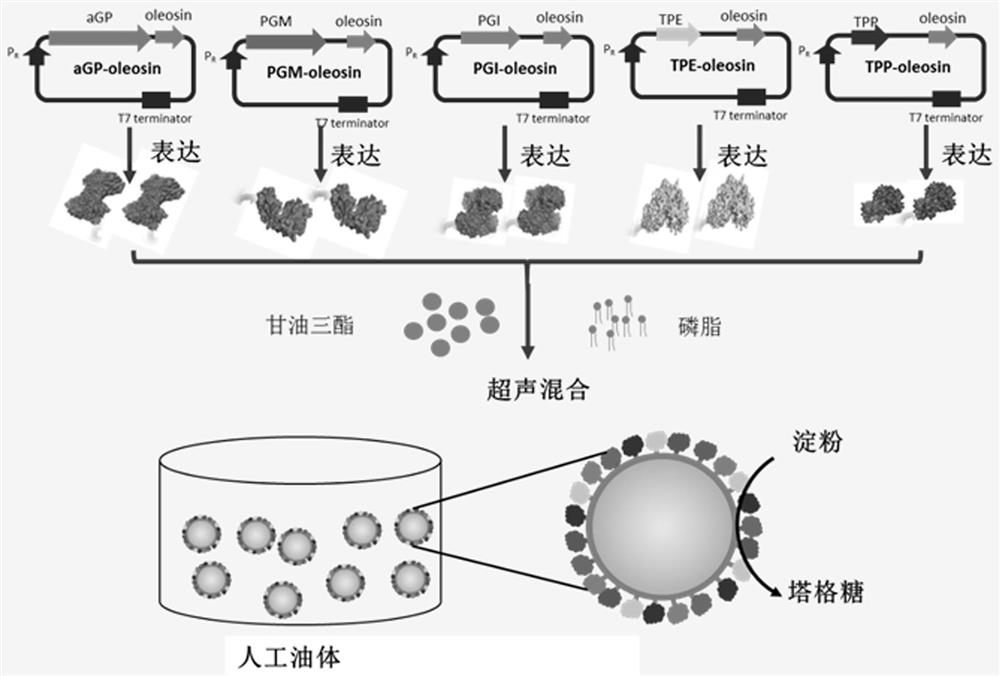
[0039] Glucan phosphorylase-oleosin fusion protein, glucose phosphomutase-oleosin fusion protein, glucose phosphoisomerase-oleosin fusion protein, 6-phosphate tagatose 4 The genes of epimerase-oleosin fusion protein and 6-phosphate tagatose phosphatase-oleosin fusion protein are constructed on the expression vector.
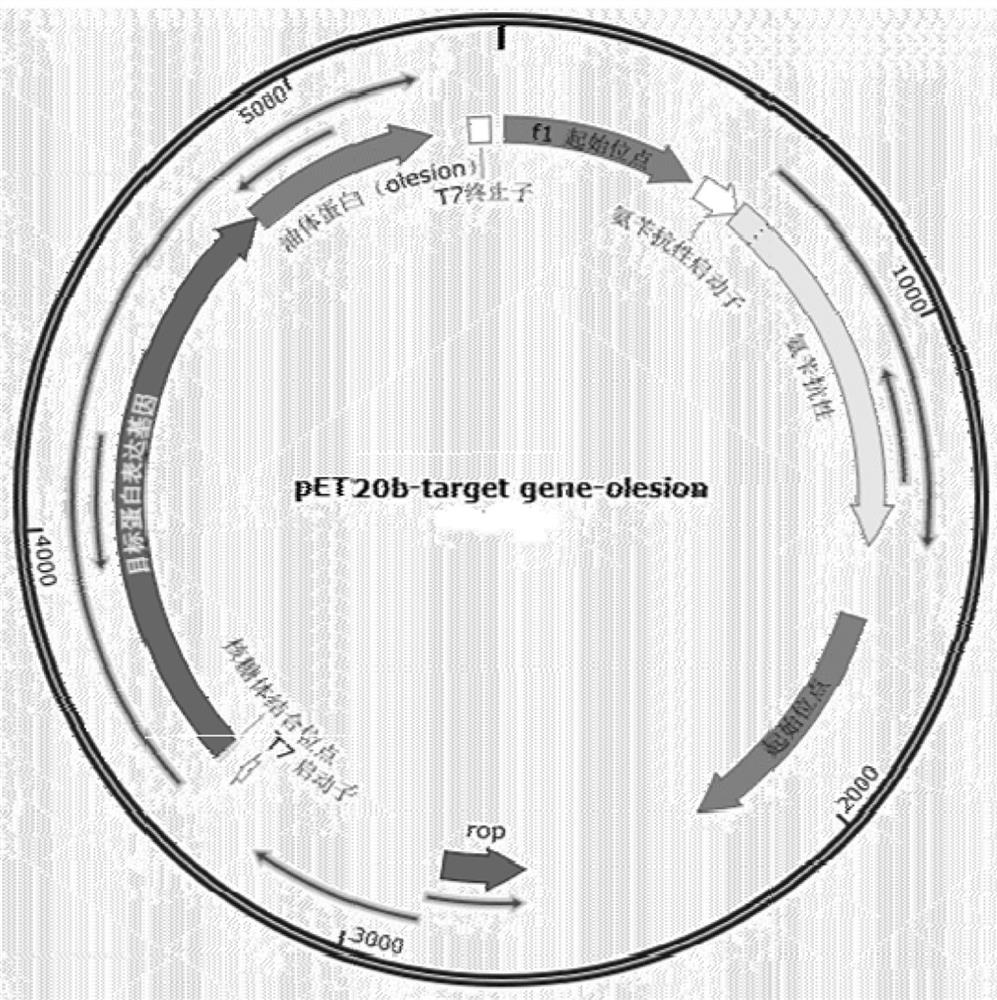
[0040] In this example, the gene encoding the first 140 amino acids of the sesame-derived olesion protein (NCBIReference Sequence: XP_011076526.1) was first codon-optimized (SEQ ID NO.1) by gene synthesis, and then synthesized into the vector pET20b vector ( The enzyme cutting sites are NdeI and XhoI) to obtain pET20b-olesion. Then, will be derived from Thermotoga maritima α-glucan phosphorylase, the number of the gene on KEGG is TM1168; derived from Pyrococcus furiosus Glucose mutase, the gene number on KEGG is PF0588; derived fr...
Embodiment 2
[0043] Example 2 Immobilized Single Enzyme in Artificial Oil Body and Detection of Enzyme Activity
[0044] 1. Artificial oil body immobilized glucan phosphorylase
[0045] according to image 3 The preparation process shown constructs the artificial oil body immobilized glucan phosphorylase of the present invention.
[0046] Add the glucan phosphorylase-oleosin fusion protein (100 mg) prepared in Example 1 into a test tube, add 75 mg triglycerides, 750 ug lecithin, and then process the sample with ultrasound (power 20-30%), centrifuge at 10,000 rpm / min after 10 min, and collect the white substance in the upper layer, which is artificial oil body-immobilized glucan phosphorylase.
[0047] 2. Artificial oil body immobilization of glucose phosphomutase: the method is the same as the above step 1, the difference is that the immobilized enzyme is changed from glucan phosphorylase to glucose phosphomutase.
[0048] 3. Artificial oil body immobilization of glucose phosphate isome...
Embodiment 3
[0059] Example 3 Production of Tagatose by Mixed Reaction of Single Enzyme Immobilized in Artificial Oil Body
[0060] Adopt each immobilized single enzyme that embodiment 2 provides, prepare tagatose by following method:
[0061] Get starch 100 g / L, the HEPES damping fluid 100 mM that pH value is 6.5, inorganic phosphate radical 40 mM, divalent magnesium ion 5 mM, zinc ion or manganese ion 0.5 mM, debranching enzyme 5 U / ml, the embodiment 2 The prepared immobilized single enzymes were mixed, wherein, glucan phosphorylase 0.1 g / L, glucose phosphomutase 0.1 g / L, glucose phosphoisomerase 0.1 g / L, 6-phosphate tagatose The 4-position epimerase was 0.2 g / L, the 6-phosphate tagatose phosphatase was 0.2 g / L, the reaction was carried out at 70°C, and the concentration of tagatose was detected by high performance liquid chromatography.
[0062] The result is as Figure 9 As shown, after 2 hours of reaction, the production concentration of tagatose was 14 g / L, and after 12 hours of re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com