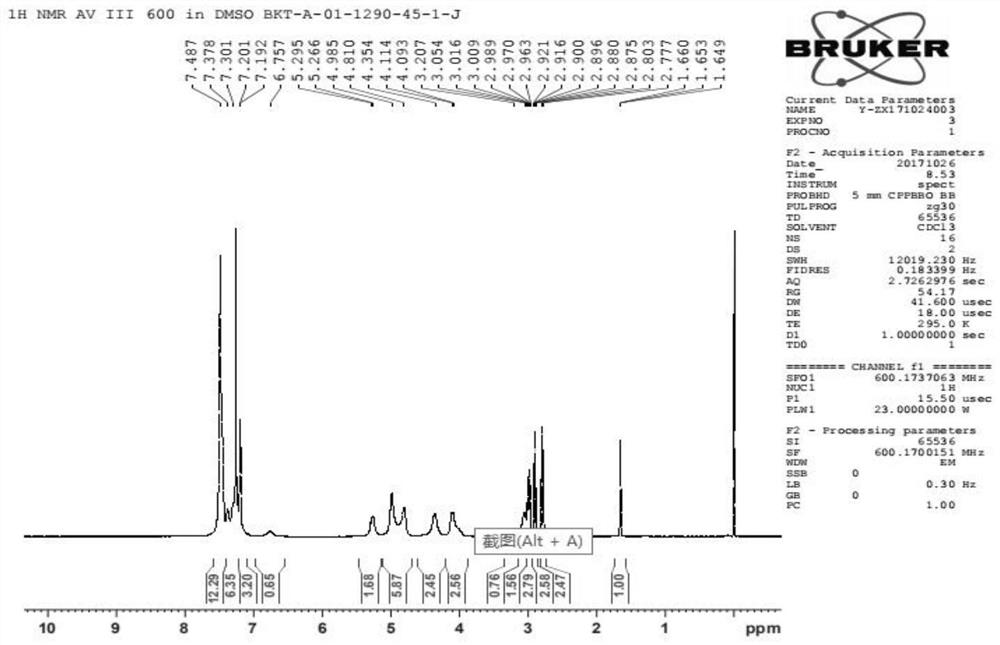
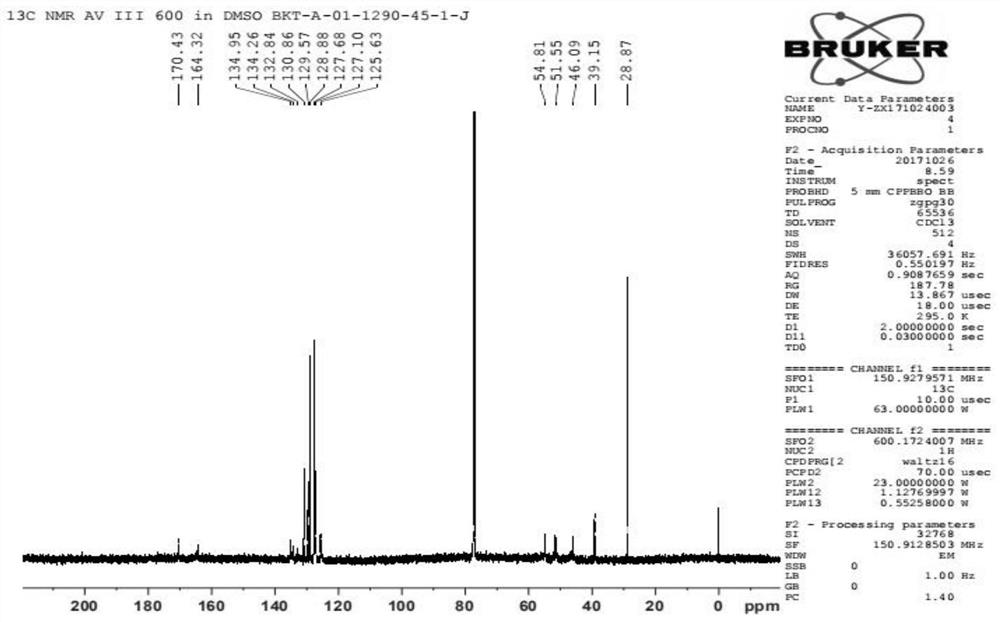
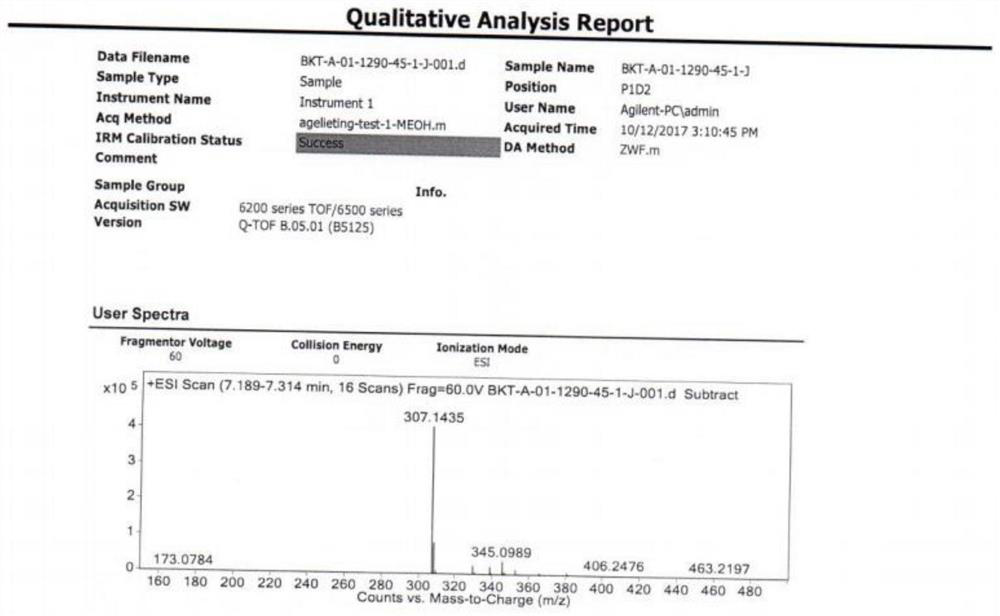
Praziquantel impurity A and preparation method thereof
A technology of praziquantel and impurities, which is applied in the field of organic compound synthesis, and can solve problems such as low conversion rate and cumbersome operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] S11. Add 100mL of water and 50mL of concentrated ammonia water to a 500mL autoclave, control the pH of the system to 10, add 40g of praziquantel, and heat up to 80°C under 0.01Mpa for reflux reaction for 5h, then cool down after the reaction to 10°C, release the pressure to obtain a feed liquid containing intermediate D;
[0049] S12. Use dichloromethane to extract three times, each time using 200mL, combine the dichloromethane layers, and use a rotary evaporator to evaporate to dryness at 40°C to obtain 1,2,3,6,7,11b-hexahydropyrazino[ 2,1-α]isoquinolin-4-one intermediate D;
[0050] S13. Add 24.0g of intermediate D to a 500mL reaction flask, add 150g of dichloromethane, add 12g of triethylamine, and add 15.0g of benzoyl chloride dropwise at 25°C. Continue to react for 3h under the condition;
[0051] S14. Add 150 mL of water to the solution after the reaction in step S13 and stir for 20 minutes, then add hydrochloric acid dropwise to adjust the pH to 2, and let stan...
Embodiment 2
[0055] S21. Add 100mL of water and sodium hydroxide to a 500mL autoclave, and control the pH value of the system to 10, add 40g of praziquantel, and raise the temperature to 80°C under the condition of 0.01Mpa, heat and reflux for 5h, after the reaction is completed Cool down to 10°C, release the pressure to obtain a feed liquid containing intermediate D;
[0056] S22. Use ethyl acetate to extract three times, each time 250 mL, combine the ethyl acetate layers, and use a rotary evaporator to evaporate to dryness at 55 ° C to obtain 1,2,3,6,7,11b-hexahydropyrazino[ 2,1-α]isoquinolin-4-one intermediate D;
[0057] S23. Add 23.2g of intermediate D to a 500mL reaction flask, add 170g of ethyl acetate, add 12g of triethylamine, and add 15.0g of benzoyl chloride dropwise at 25°C. Continue to react for 3h under the condition;
[0058] S24. Add 150 mL of water to the solution after the reaction in step S23 and stir for 20 minutes, then add sulfuric acid dropwise to adjust the pH to ...
Embodiment 3
[0062] S31. Add 100mL of water and 45mL of concentrated ammonia water to a 500mL autoclave, and control the pH of the system to 9, add 40g of praziquantel, and raise the temperature to 90°C under 0.02Mpa for reflux reaction for 3h. After the reaction is completed Cool down to 20°C, release the pressure to obtain a feed liquid containing intermediate D;
[0063] S32. Extract three times with dichloromethane, each time 200 mL, combine the ethyl acetate layers, and evaporate to dryness at 45° C. with a rotary evaporator to obtain 1,2,3,6,7,11b-hexahydropyrazino[ 2,1-α]isoquinolin-4-one intermediate D;
[0064] S33. Add 24.5g of intermediate D to a 500mL reaction flask, add 160g of dichloromethane, add 11g of triethylamine, and add 12.6g of benzoyl chloride dropwise at 25°C. Continue to react for 3h under the condition;
[0065] S34. Add 150 mL of water to the solution after the reaction in step S33 and stir for 40 minutes, then add hydrochloric acid dropwise to adjust the pH to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com