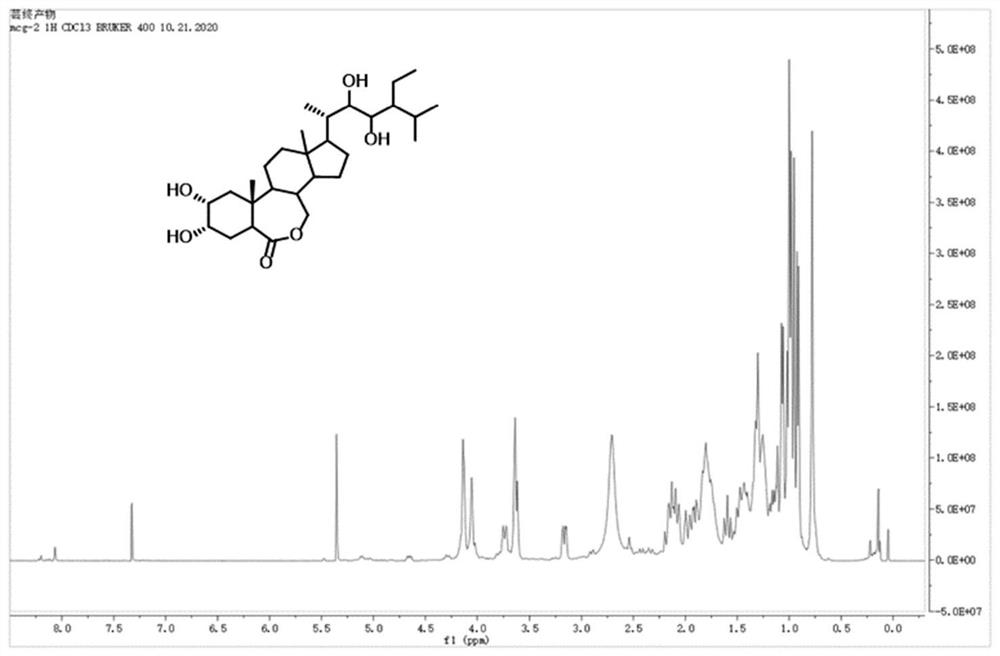
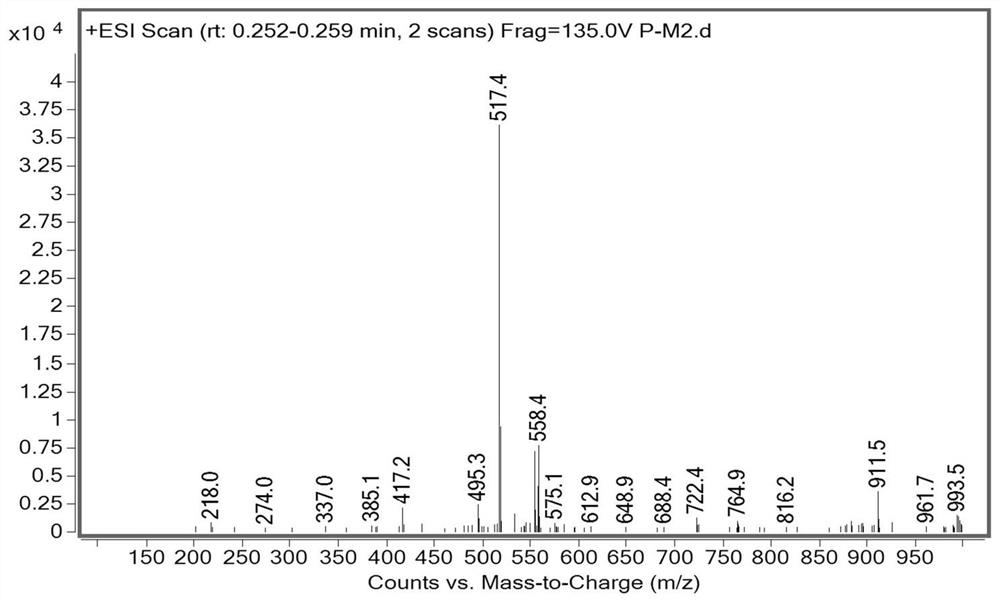
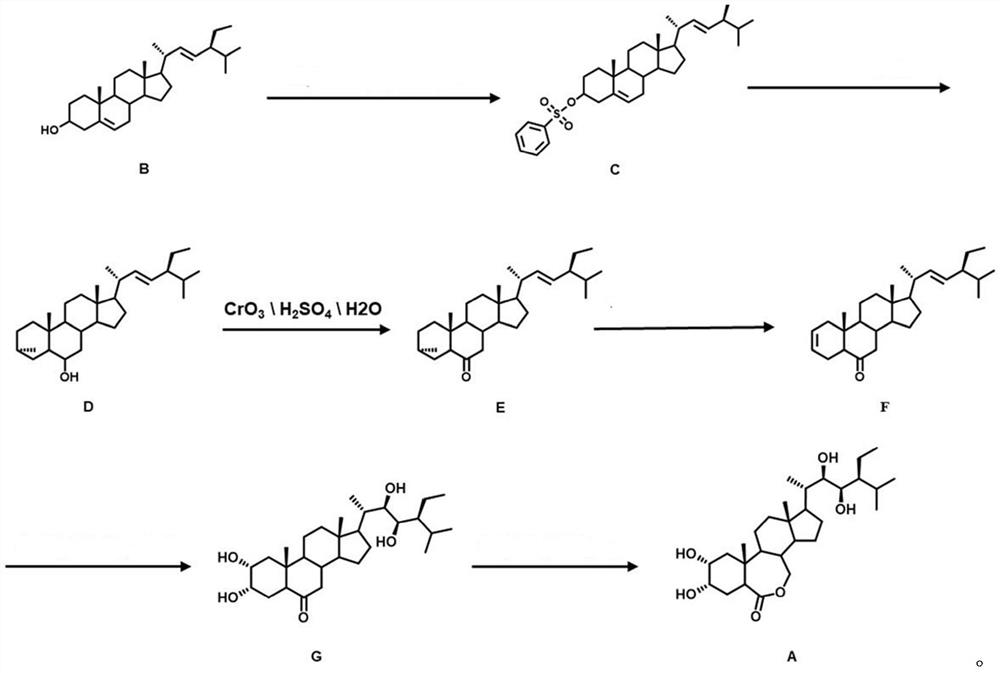
Synthesis method of 28-homobrassinolide
A synthesis method and a technology for sterolide, which are applied in the fields of botanical equipment and methods, steroids, chemicals used for biological control, etc., can solve the problems of complicated operation, low yield, pollute the environment, etc., and achieve a synthesis method. Simple, simple process, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036](1) (5,22) - Preparation of diene-3-methylsulfyl ester (C)
[0037]In an ice bath at 3 ° C, 8.2 g (20 mmol) of elthyl alcohol was dissolved in 100 ml butanone, 6.2 ml (4.04 g, 40 mmol) triethylamine was added. Under stirring, 3.44 g (30 mmol) of toluenesulfonyl chloride was slowly added dropwise, and the reaction was continued for 1 h. TLC dotted tracks, after completion of the reaction, 40 ml of NaCl solution was quenched. The standing hierarch is divided into the upper organic phase, and the organic phase sequentially uses 40ml NaHCO.3The solution was washed twice with 100 mL of water. The organic phase was poured into a beaker with 800 ml of ice water, stirred for 20 min, put it into the refrigerator crystallization for 15 min, filtered, filter cake was washed with ice water, and the filter cake was placed in an oven to dry, 9.53 g of intermediate C ( 5,22) - Dienne-3-methylsulfamatide crude, yield 97%.
[0038](2) Preparation of 22E-Intene-3α, 5-ring-5α cholester-6-alcohol (D)
[0...
Embodiment 2
[0051]Example 2 was the same as that of the first embodiment, and the preparation of 22E-lyne-3α, 5-ring -5α cholester-6-alcohol (D): the resulting 4.90 g (10 mmol) intermediate C is dissolved in 100 mL of acetone, add 20 ml of water and 1.84 g (10 mmol) kHCO to the flask.3Heated at 75 ° C to reflux, reaction for 5 h. The TLC point plate tracking, after the reaction is complete, cool down, stand in the hierarchy, and separate the organic phase. The organic phase was washed twice with 40ml NaCl; then, with anhydrous NaHSO4Dry, filtered, vacuum yarn. The crude product was separated by column chromatography. The intermediate 22e-olene-3α, 5-ring-5α cholester-6-alcohol 3.07 g, yield of 78%.
Embodiment 3
[0053]Example 3 is the same as that of the preparation method of Example 1, and the preparation of 22E-anne-3α, 5-ring-5α cholester-6-alcohol (D): the resulting 4.90 g (10 mmol) intermediate C is dissolved in 100 ml of acetone, add 20 ml of water and 2.45 g (20 mmol) Na to the flask.2CO3Heated at 75 ° C to reflux, reaction for 5 h. The TLC point plate tracking, after the reaction is complete, cool down, stand in the hierarchy, and separate the organic phase. The organic phase was washed twice with 40ml NaCl; then, with anhydrous NaHSO4Dry, filtered, vacuum yarn. The crude product was separated by column chromatography. The intermediate 22e-olene-3α, 5-ring-5α cholester-6-alcohol 2.98 g, yield of 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com