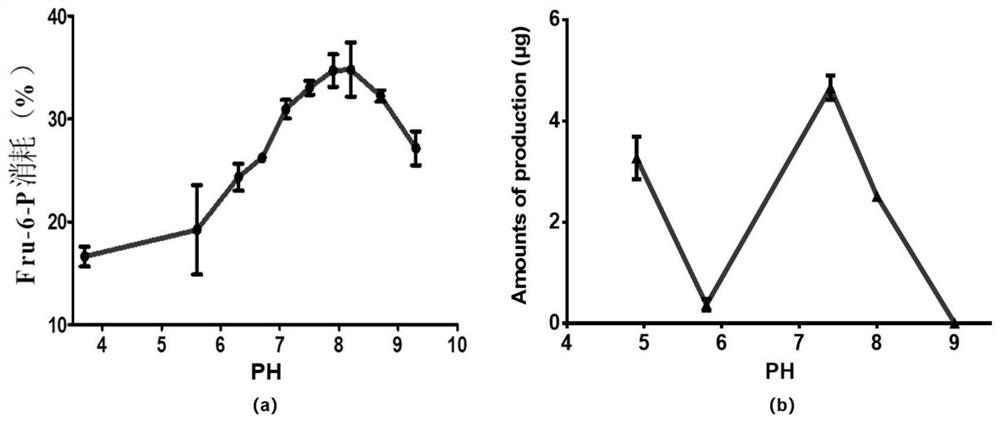
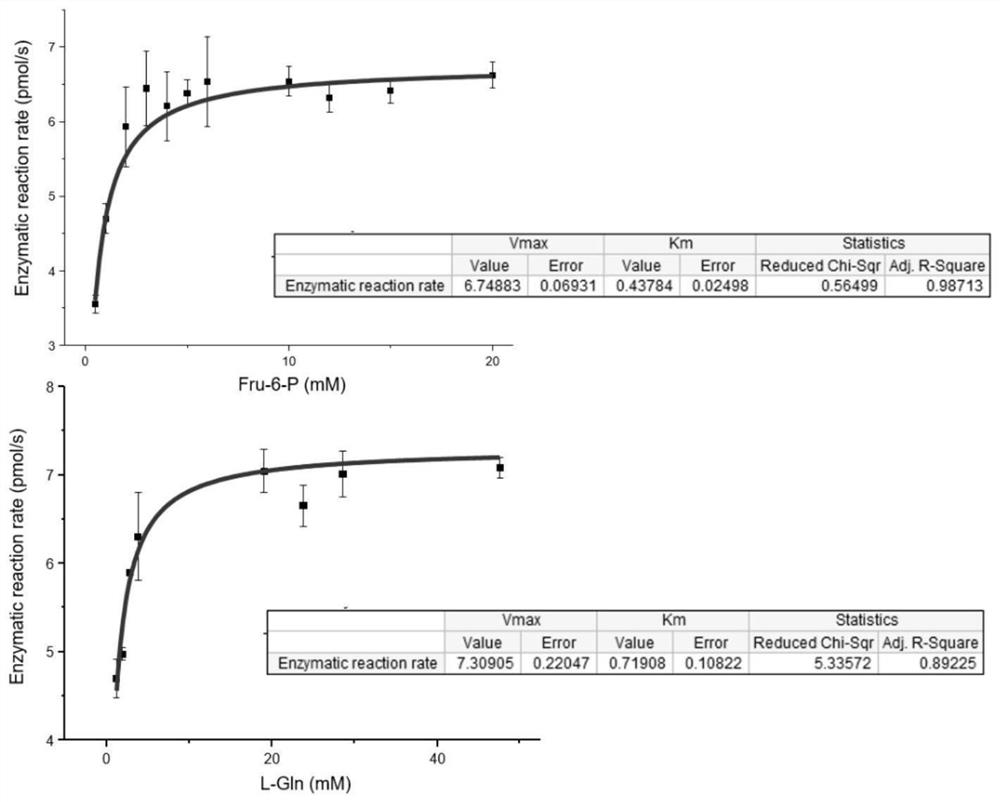
Preparation and application of glutamine-fructose-6-phosphate aminotransferase coding gene and glutamine-fructose-6-phosphate aminotransferase enzyme
A technology of aminotransferase and fructose phosphate, applied in the direction of transferase, application, genetic engineering, etc., can solve the problem that GFAT has no relevant transformation research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Cloning of embodiment 1 phosphofructose aminotransferase OsGFAT full-length gene
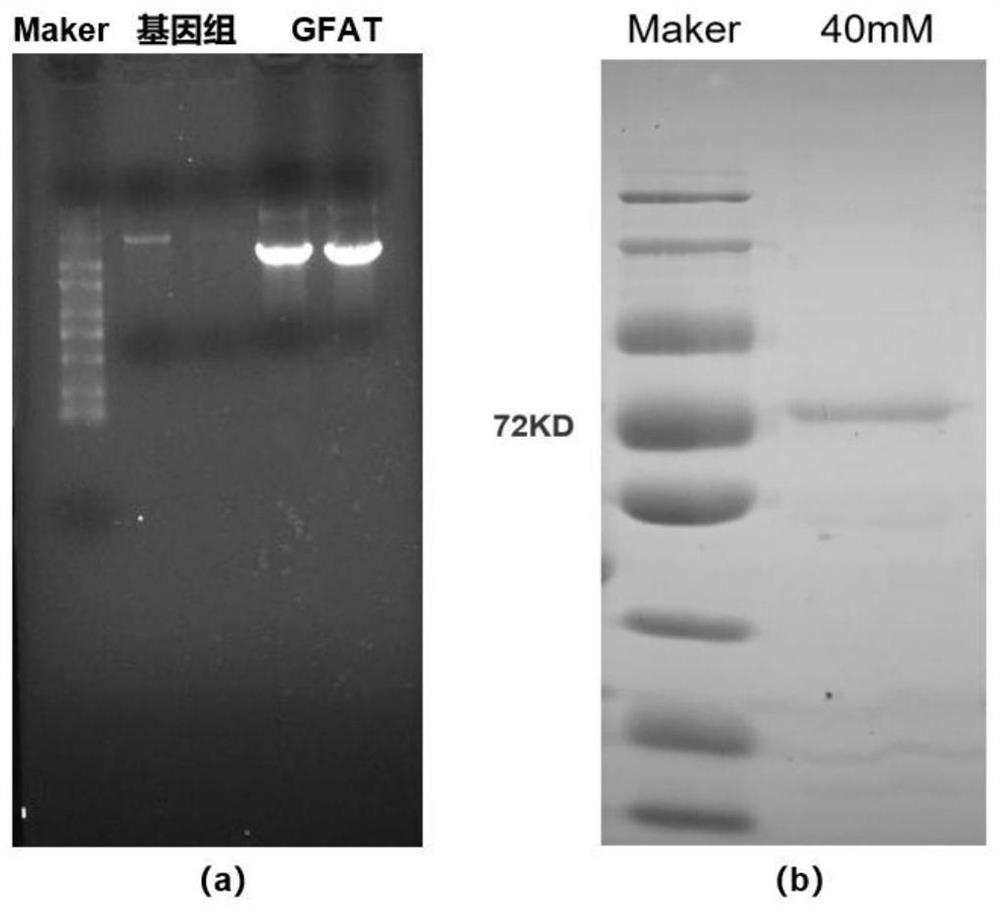
[0051] The mRNA of rice leaves was extracted according to the operating steps of the RNA extraction kit (Bomad Biology, Cat. No. RN0112). After analyzing the sequence of phosphofructose aminotransferase in The National Center for Biotechnology Information (NCBI) database, design primers GFAT-F: 5'-CATTCCATGGATATGTGCGGGATCTTCGCG-3'; GFAT-R: 5'-CGGTAAGCTTTTGGGTAGTCACACTCTTTGCC-3' to amplify the phosphate The gene sequence of the mature protein of fructose aminotransferase OsGFAT was amplified by PCR using the reversed cDNA of the extracted rice RNA as a template. The PCR reaction conditions were: 94°C for 2 min, 1 cycle; 94°C for 30 s, 58°C for 30 s, 72°C for 3 min, 35 cycles; 72°C for 5 min, 1 cycle. PCR products were analyzed by agarose gel electrophoresis (eg figure 1 (a)), the target fragment was recovered by gel cutting, ligated to the prokaryotic expression vector pET28a after doubl...
Embodiment 2
[0052] Embodiment 2 Phosphofructose aminotransferase OsGFAT gene sequence analysis
[0053]The results of the sequencing were analyzed using the Basic Local Alignment Search Tool (BLAST) in the GenBank database, and the Vector NTI Suite 8.0 software was used for multiple sequence alignment and sequence information analysis.
[0054] The coding region of the obtained phosphofructose aminotransferase gene (named OsGFAT) is 2043bp long, and its nucleotide sequence is shown in SEQ ID NO 1. OsGFAT encodes 679 amino acids and a stop codon, its amino acid sequence is shown in SEQID NO 2, the theoretical protein molecular weight is 74.41kDa, and the predicted isoelectric point is 6.1. It is predicted by bioinformatics that the protease expressed by this gene may have the function of phosphofructose aminotransferase, but it has not been studied so far.
Embodiment 3
[0055] Example 3 Recombinant expression and purification of OsGFAT gene in Escherichia coli
[0056] Sequencing results showed that the OsGFAT gene shown in SEQ ID NO 1 was inserted into pET28a, and the insertion direction was correct, which proved that the constructed recombinant plasmid was correct, and the recombinant plasmid was named pET28a-OsGFAT.
[0057] Transform pET28a-OsGFAT into Escherichia coli strain BL21(DE3) for induced expression. Add the overnight cultured seed solution to fresh LB medium at 37°C with an inoculation amount of 1% to carry out expanded cultivation. When the OD600nm of the bacterial solution = 0.6-0.8, add IPTG to make the final concentration 0.25mM, and carry out at 10°C 72h induction. After the cells were broken, the supernatant was collected by high-speed centrifugation and purified by a nickel column, and eluted with gradient imidazole (20-250mM imidazole, 20mM Tris-HCl, pH7.1). Polyacrylamide gel electrophoresis was used to detect the exp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com