Method for synthesizing nicotinamide mononucleotide based on enzyme method
A single nucleotide and enzymatic synthesis technology, applied in the direction of fermentation, etc., can solve the problems of limited production and application, high product impurities, environmental pollution, etc., and achieve the effect of low cost, high product purity and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The construction of embodiment 1 enzyme gene expression engineering strain
[0045] The ribokinase, phosphoribosyl mutase and nicotinamide ribokinase amino acid sequences involved in the present invention were sent to Gene Synthesis Company for codon optimization of Escherichia coli and artificially synthesized coding genes, respectively cloned into NdeI of the prokaryotic expression vector pET30a(+) Between the XhoI restriction site and the C-terminus of the above genes, 6 histidine coding sequences are added to facilitate subsequent protein purification. The recombinant expression vectors containing the above three enzyme genes were introduced into Escherichia coli BL21(DE3) by electrotransformation to obtain corresponding engineering strains for enzyme gene expression. The fermentation of engineering strains adopts conventional fermentation medium TB, and first cultivates OD at 37°C and 200rpm. 600 reach 0.6-0.8, and then use the final concentration of 0.5mM IPTG or...
Embodiment 2
[0046] The preparation of embodiment 2 immobilized enzyme
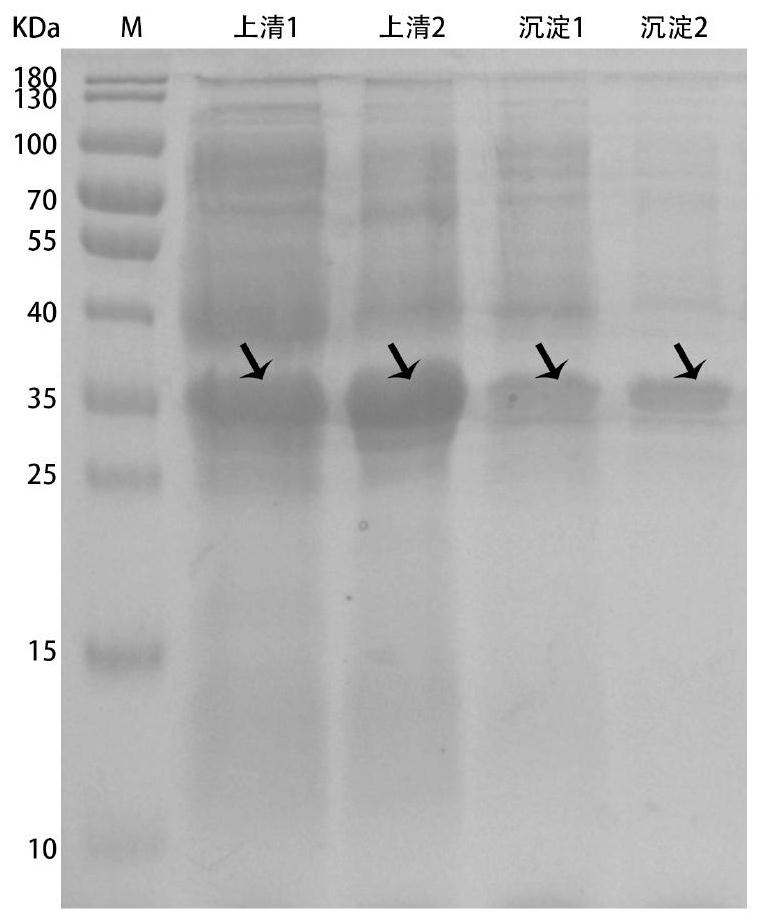
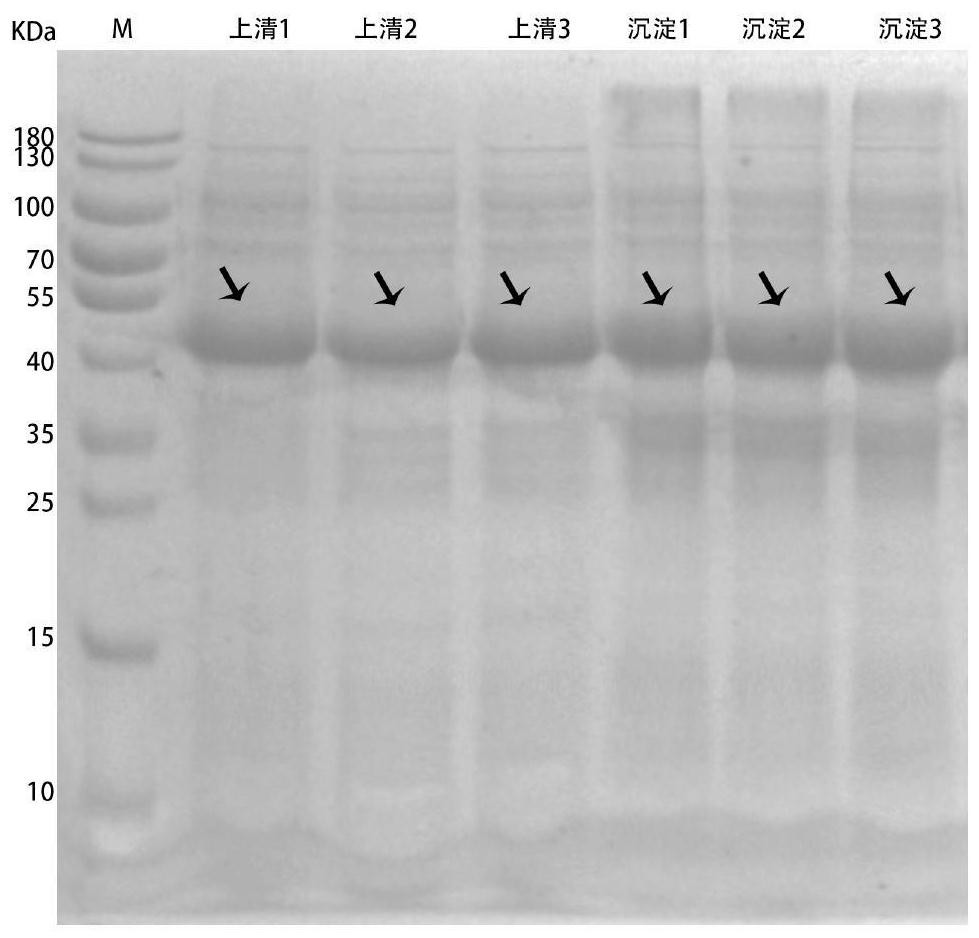
[0047] Use a low-temperature high-speed centrifuge (10000rpm, 4°C, 10min) to collect ribokinase, phosphoribosyl mutase and nicotinamide ribokinase fermentation cells after induction for 10 hours, and wash repeatedly with pH 8.0, 0.1mol / L phosphate buffer Bacteria were resuspended 20 times concentrated in 50ml of phosphate buffer, and ultrasonically disrupted in an ice bath (ultrasonic conditions: working 2s, interval 5s) until clarified. Centrifuge the above broken solution in a low-temperature high-speed centrifuge (10000rpm, 4°C, 20min), collect the supernatant to obtain the crude enzyme solutions of ribokinase, phosphoribosylmutase and nicotinamide ribokinase, and then use vacuum freeze-drying to prepare into crude enzyme protein powder. Further inject the above crude enzyme protein solution onto the IDA resin that has been activated and bound to Ni+, first use 1-2 column volumes of 10 and 30mM low-concentration i...
Embodiment 3
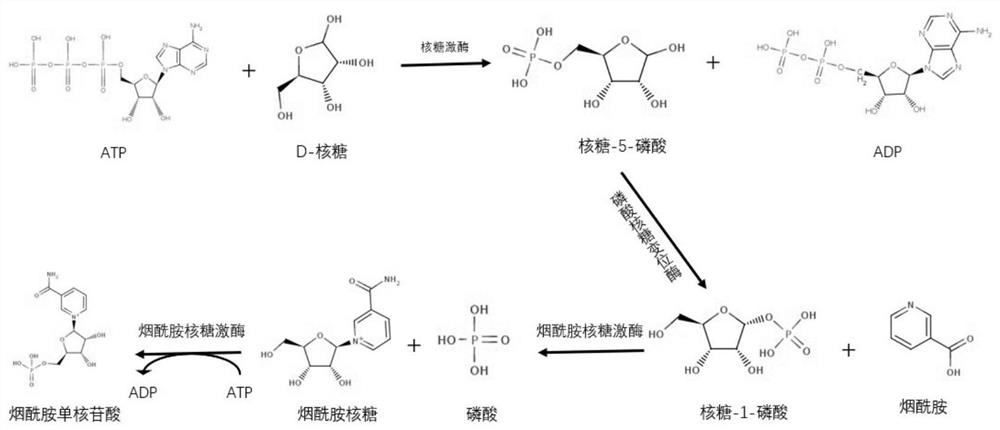
[0048] Example 3 Using D-ribose as a substrate, using crude enzyme one-pot method to produce NMN
[0049] Add D-ribose with a final concentration of 50mM, 50mM nicotinamide, 60mM ATP, 5mM manganese chloride, and 20mM magnesium chloride in sequence to the reaction system, and use 1L of pH 6.0 and 50mM phosphate buffer solution to fully shake and dissolve, then adjust the pH to 5.00 . Add 300 mg of ribokinase crude enzyme freeze-dried powder prepared in Example 2, 300 mg of nicotinamide ribokinase crude enzyme freeze-dried powder, and 600 mg of phosphoribosylmutase crude enzyme freeze-dried powder, mix well, and control the reaction temperature to 25 ° C. 300rpm stirred reaction, adopts automatic titrator, controls pH 5.0 with 1mol / l sodium hydroxide in the whole process, detects the generation concentration of NMN with liquid chromatography HPLC in the reaction process, reacts and finishes in 4 hours, and reaction obtains NMN 14.8g, and reaction yield The yield was 88.62%. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com