Application of 3-indoleacetonitrile in the preparation of drugs for inhibiting novel coronavirus sars-cov-2
A technology of indole acetonitrile and sars-cov-2, applied in antiviral agents, drug combinations, pharmaceutical formulations, etc., can solve problems such as storms, reduce the mortality rate of new crowns, and have no anti-SARS-CoV-2, etc., to achieve weight loss Degree of decline, large clinical therapeutic potential, effect of reducing viral load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
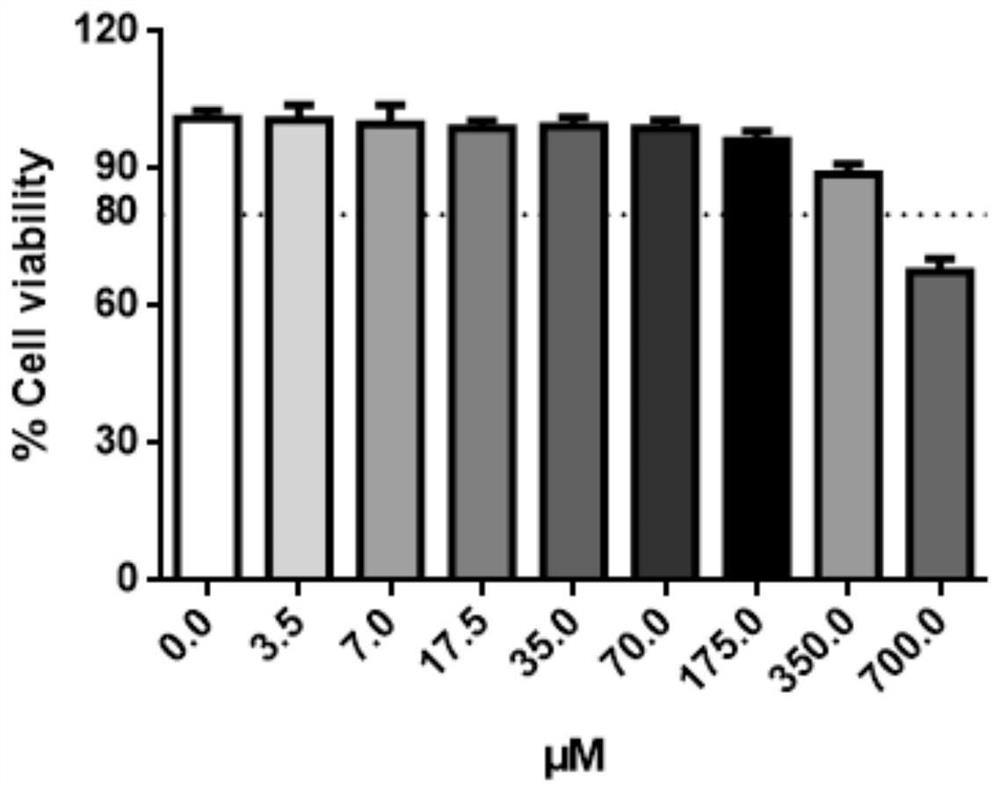
[0049] Toxicity test of 3-indoleacetonitrile to cells
[0050] Use Caco-2 cell among the present invention, inoculate 96 well plate, treat that cell density reaches 80%, with 3-indole acetonitrile (final concentration is: 0 μ M, 3.5 μ M, 7 μ M, 17.5 μ M, 35 μ M, 70 μ M, 175 μ M, 350 μ M, 700 μM) to incubate the cells for 48 hours. Cell viability was measured using TransDetect cell counting box, and the absorbance at OD450nm was measured according to the instructions of the kit. The specific implementation process is as follows:
[0051] 1. Cell culture
[0052] The frozen and revived Caco-2 cells were subcultured twice, and expanded with DMEM medium containing 10% fetal bovine serum and double antibodies (penicillin 100 U / ml, streptomycin 100 ug / ml).
[0053] 2. Toxicity test of 3-indoleacetonitrile to cells
[0054] Well-grown Caco-2 cells were taken for digestion and passage, and the cell density was adjusted to 2×10 with cell growth medium (DMEM medium + 10% fetal bovin...
Embodiment 2
[0059] Inhibitory effect of 3-indoleacetonitrile on novel coronavirus at the cellular level:
[0060] Western Blot and plaque forming unit (plaque forming unit) were used to detect the influence of 3-indoleacetonitrile on the proliferation of SARS-COV-2 virus (WBP-1) at the cellular level, and the specific steps were as follows:
[0061] 1. 3-indoleacetonitrile treated cells and infected the new coronavirus WBP-1
[0062] 1) The Caco-2 cells in good growth state were digested and passaged, and the cell density was adjusted to 1×10 with cell culture medium. 5 / ml, 1ml per well was inoculated in a 12-well plate to grow to a monolayer.
[0063] 2) Add different amounts of 3-indoleacetonitrile, the final concentrations of 3-indoleacetonitrile are 0 μM, 75 μM, 150 μM, and 300 μM, respectively, and the working concentration of 0 μM is the no drug control.
[0064] 3) After being treated with different concentrations of 3-indoleacetonitrile for 24 hours, the cells were infected wit...
Embodiment 3
[0088] Evaluation of antiviral effect of 3-indoleacetonitrile in lethal infection model of mice
[0089] Mouse experiment steps:
[0090] 1) The 6-week-old female BALB / c mice were randomly divided into 2 large groups. The first large group was the non-infected new coronavirus group, which were the control group (PBS) (5 mice), the 3-indoleacetonitrile group ( 5 rats); the second largest group is the SARS-CoV-2 virus infection group, respectively PBS+SARS-CoV-2 infection group (10 rats), 3-indoleacetonitrile+SARS-CoV-2 infection group (10 rats ).
[0091] 2) 3-indoleacetonitrile was prepared as a 4 mg / mL aqueous solution, and each mouse was injected with 100 μL of PBS or 3-indoleacetonitrile solution through the tail vein each time.
[0092] 3) 2 hours before infection with SARS-CoV-2, inject 3-indoleacetonitrile (3-indoleacetonitrile+SARS-CoV-2 infection group) or PBS (PBS+SARS-CoV-2 infection group) once into the tail vein, and then Carry out intranasal infection 5 median ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com