Electrochemical method for preparing high-purity molybdenum disulfide nanosheet from molybdenite
A molybdenum disulfide and molybdenite technology, which is applied in the field of metallurgical engineering, can solve the problems of high ore raw material grade requirements, complex process, and unguaranteed performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
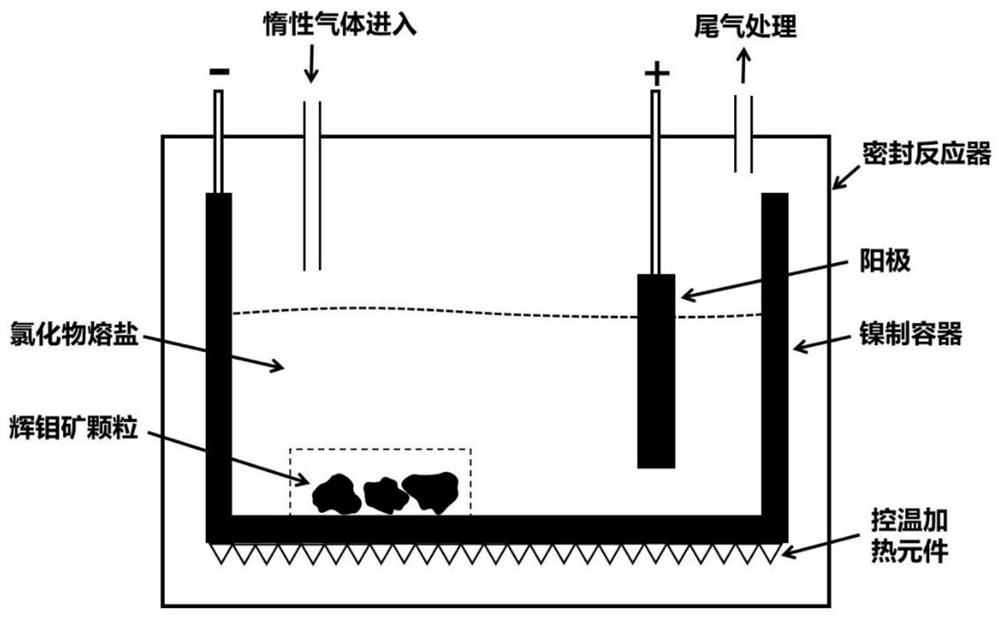
[0035] (1) the mixture of the NaCl-KCl (50:50mol%) of 1000g is filled in the nickel container of suitable size, then one end of a molybdenum rod is connected with the nickel container, and the other end is stretched out outside the reactor and connected with Connect to the cathode of the external power supply. The nickel container is slowly heated to 800°C in an electric furnace under the protection of an argon atmosphere (the heating rate does not exceed 20°C / min) to melt the chloride salt, and then reduce the temperature of the molten salt to 700°C within 30 minutes.
[0036] (2) The molybdenite ore with a molybdenum content of about 46.33% is mechanically crushed, and screened to obtain molybdenite particles with a particle size below 20mm; select a cylindrical graphite anode, and screen the obtained molybdenite particles (mass about 10g) Put it into a nickel container, and carry out constant voltage electrolysis (electrolyzer voltage: 2.9V) under an argon atmosphere, and t...
Embodiment 2
[0040] (1) Fill the mixture of 700g of NaCl-KCl (45:55mol%) into a nickel container of suitable size, then connect one end of a nickel rod with the nickel container, and the other end stretches out of the reactor And connected to the cathode of the external power supply. The nickel container was slowly heated to 800°C in an electric furnace under the protection of an argon atmosphere to melt the chloride salt, and then the temperature of the molten salt was adjusted to 685°C within 40 minutes.
[0041] (2) The molybdenite ore with a molybdenum content of about 41.57% is mechanically crushed and screened to obtain molybdenite particles with a particle size of 5 to 20 mm; select a flat stainless steel-based molybdenum disulfide coated anode (size is 5 × 5cm 2 ), put the screened molybdenite particles into a nickel container, and perform constant voltage electrolysis (electrolyzer voltage: 2.6V) under an argon atmosphere, and the electrolysis time is 5.0h.
[0042] (3) After the...
Embodiment 3
[0044] (1) The NaCl of 800g is filled in the nickel container of suitable size, then one end of a nickel-chromium alloy rod is connected with the nickel container, and the other end stretches out of the reactor and is connected with the cathode of the external power supply. The nickel container was slowly heated to 850°C in an electric furnace under the protection of an argon atmosphere to melt NaCl.
[0045] (2) Mechanically crush the molybdenite ore with a molybdenum content of about 35%, and screen to obtain molybdenite particles with a particle size below 10 mm; select a cylindrical graphite anode, and put the screened molybdenite particles into a nickel container , Constant current electrolysis (current density of 1500mA / 1g molybdenite) was carried out under an argon atmosphere.
[0046] (3) After the electrolysis, the anode deposition product was collected, washed and dried to obtain molybdenum disulfide nanosheet powder with a purity of about 99.2% (the mass fraction of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com