Nanoscale ruthenium dioxide-coated ruthenium-loaded carbon micron sheet, and preparation method and application thereof
A technology of ruthenium dioxide and micron flakes, applied in the direction of electrolysis components, electrodes, electrolysis process, etc., can solve the problems of less active sites, poor conductivity, increase the complexity and cost of electrolyzed water devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The invention provides a preparation method of nanoscale ruthenium dioxide-coated ruthenium-loaded carbon microsheets, comprising: S1) dispersing ruthenium salt and organic carbon source in an alcohol solvent, after mixing, performing solid-liquid separation to obtain a precursor The organic carbon source contains nitrogen; or the ruthenium salt and the organic carbon source are mixed and ground to obtain a precursor; S2) the precursor is calcined at a high temperature in a reducing atmosphere to obtain an intermediate product; S3) the intermediate The product is calcined at a low temperature in an air atmosphere to obtain nanoscale ruthenium dioxide-coated ruthenium-loaded carbon microsheets.
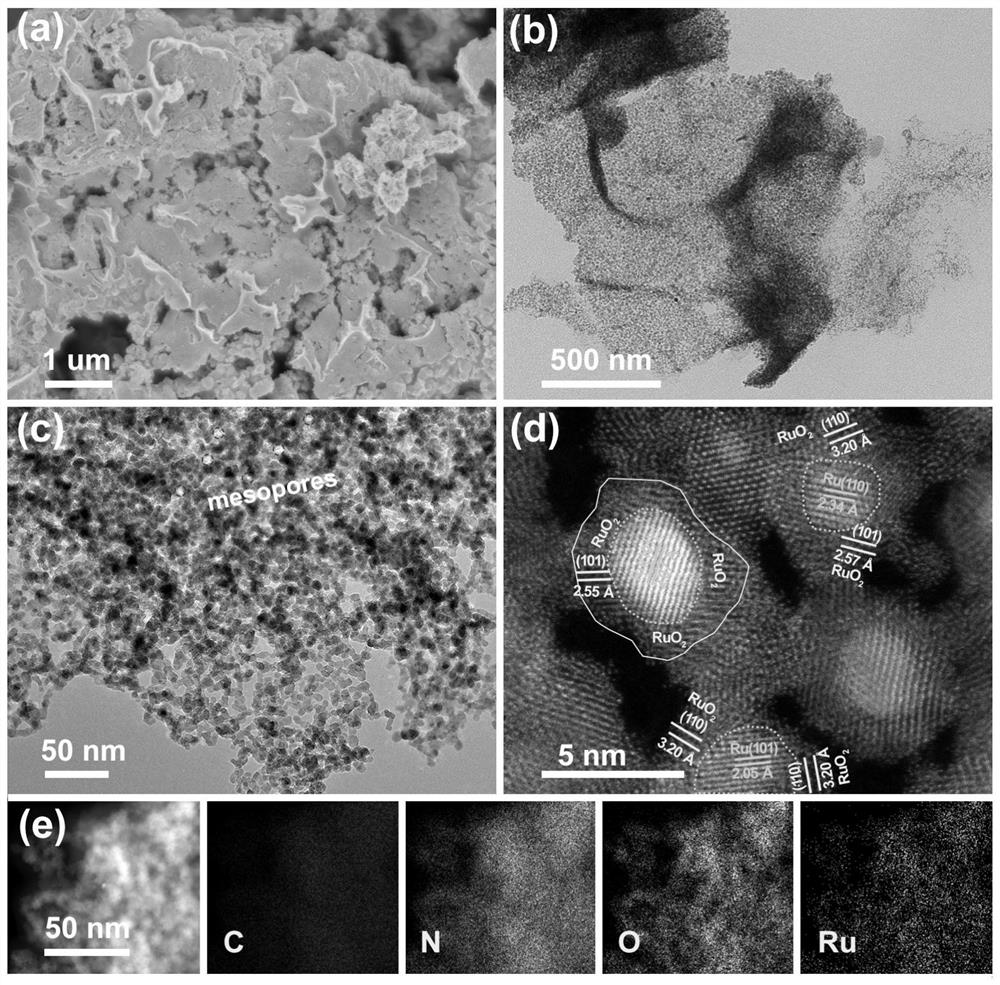
[0050] The nano-scale ruthenium dioxide-coated ruthenium-loaded carbon microsheets provided by the present invention can be produced only by simple adsorption, followed by high-temperature calcination and low-temperature air annealing. The synthesis method is relatively simple, t...
Embodiment 1
[0067] The ruthenium source is ruthenium trichloride, the organic carbon source is melamine, and the molar ratio is 1:15.
[0068] (1) Disperse ruthenium trichloride and melamine in ethanol at a molar ratio of 1:15, stir for 30 minutes, centrifuge at 8000 rpm for 2 minutes, and dry at 60°C overnight to obtain a precursor.
[0069] (2) Place the precursor in (1) in a tube furnace, raise it to 850°C at 2°C / min in an argon-hydrogen mixed atmosphere with a hydrogen volume content of 10%, and keep it for 2h, then naturally cool to room temperature, An intermediate product is obtained.
[0070] (3) Place the intermediate product obtained in (2) in a muffle furnace, raise it to 200°C at 2°C / min, and keep it for 3h, then naturally cool to room temperature to obtain the final product: nano-scale ruthenium dioxide coated ruthenium Loaded carbon microflakes.
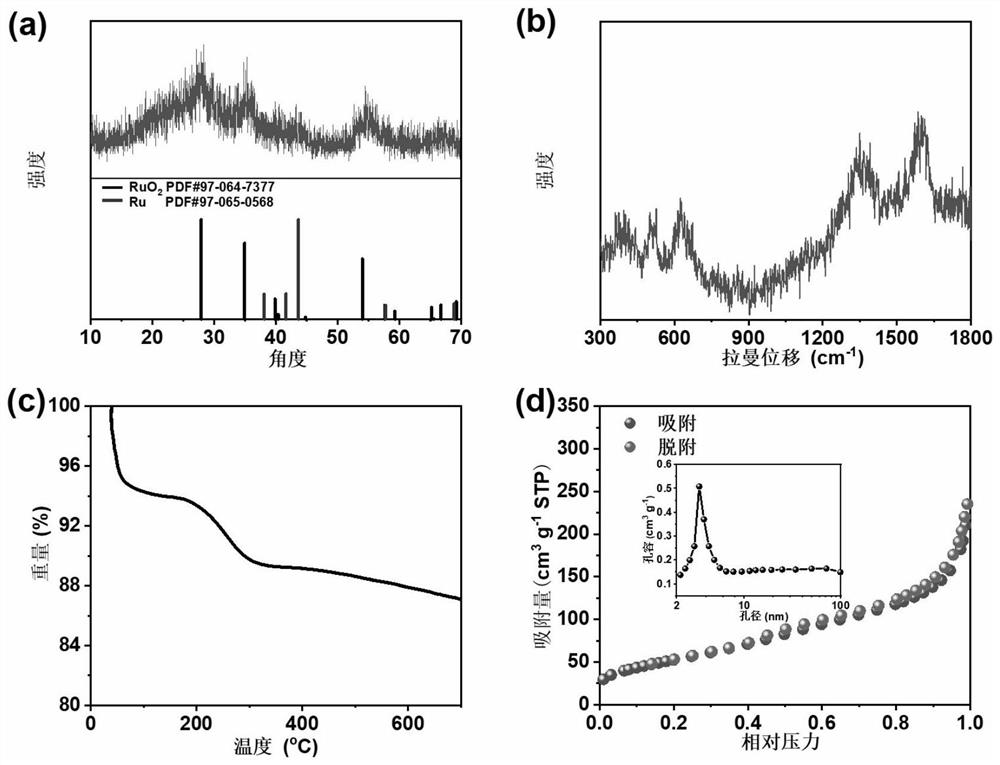
[0071] The nanoscale ruthenium dioxide-coated ruthenium-loaded carbon microsheet obtained in Example 1 is analyzed by X-ray dif...
Embodiment 2
[0086] The preparation method is the same as in Example 1, except that the heat treatment time in the muffle furnace is changed to 1h.
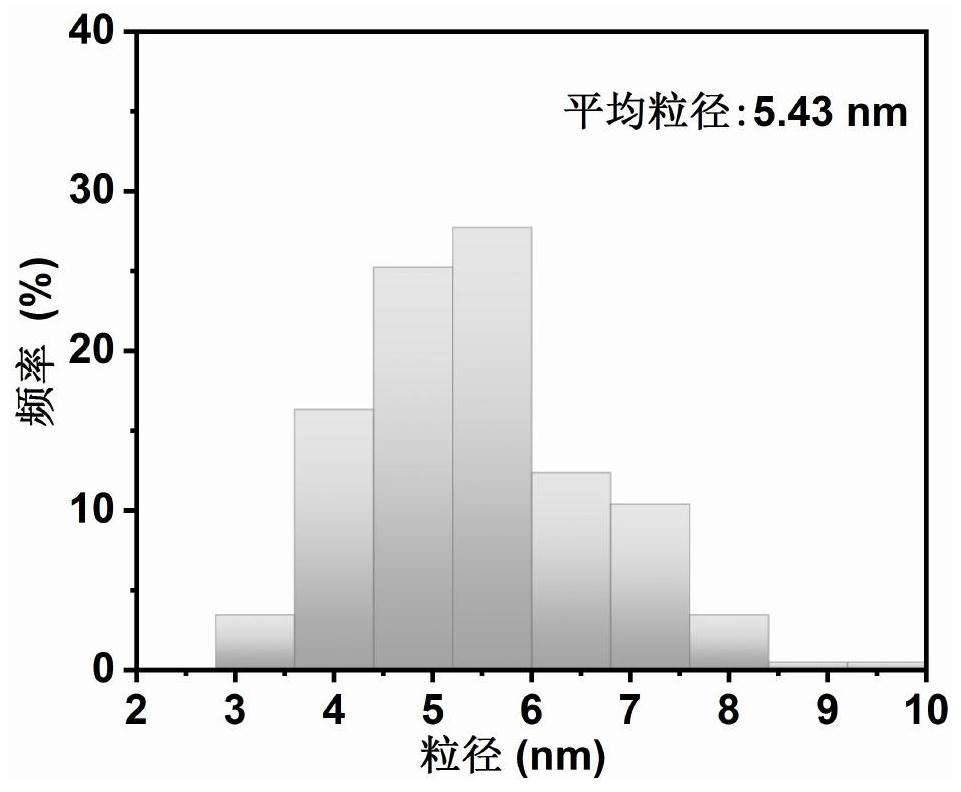
[0087] The nanoscale ruthenium dioxide-coated ruthenium-loaded carbon microsheets obtained in Example 2 are analyzed by transmission electron microscope, and its transmission electron microscope diagram is obtained as Figure 8 shown. Depend on Figure 8 It can be seen that the synthesized material is a loose and porous microsheet structure composed of nanoparticles.
[0088] Utilize X-ray diffractometer to analyze the nanoscale ruthenium dioxide-coated ruthenium-loaded carbon microsheet obtained in embodiment 2, obtain its XRD pattern, as Figure 9 shown. Depend on Figure 9 It can be seen that the characteristic diffraction peaks of ruthenium dioxide and ruthenium exist simultaneously.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com