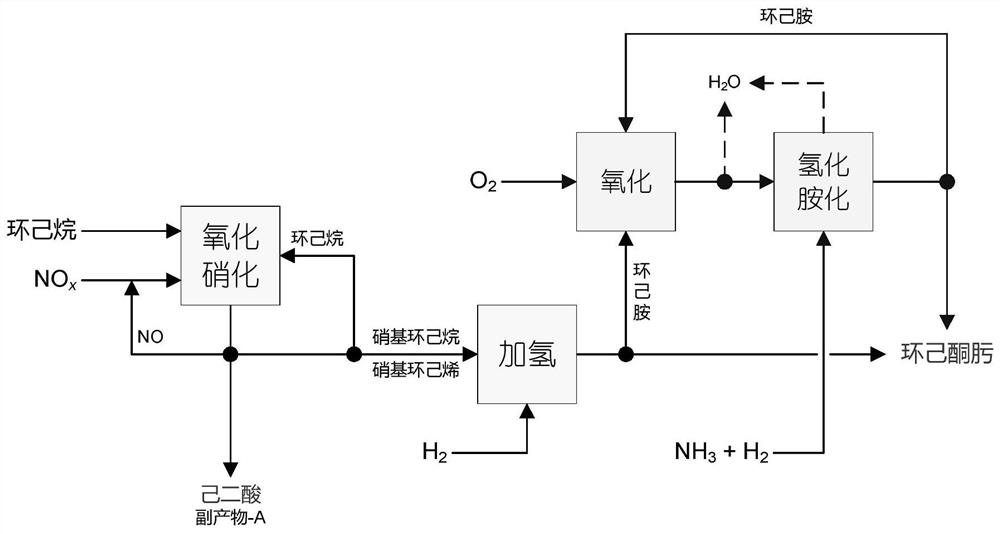
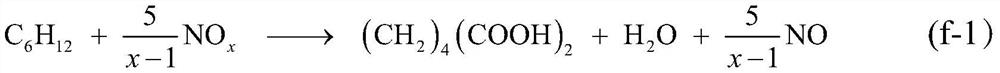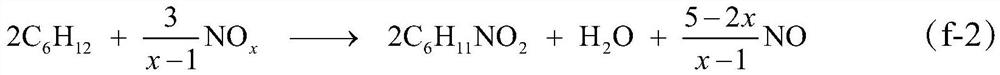Method for co-producing adipic acid and cyclohexanone-oxime from cyclohexane
A technology of cyclohexanone oxime and cyclohexane, which is applied in the field of preparation of adipic acid or cyclohexanone oxime, can solve the problems of high energy consumption, difficulty in realizing large-scale production, serious environmental pollution, etc., and achieves low material consumption and energy consumption , strong environmental friendliness, environmental friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: adopt fixed bed gas phase continuous reaction process. The liquid cyclohexane is input by the metering pump, and after being vaporized in the preheating section, the cyclohexane:NO 2 The molar ratio of 0.2:1, cyclohexane and NO 2 The mixture passes through a glass tube reactor with an inner diameter of φ10, which is filled with a V with a height of about 10 cm 2 o 5 / MCM-41 catalyst; the temperature of the reactor is controlled at 180°C, and the outlet gas of the reactor is condensed through a glass condenser tube with a cooling jacket and then evacuated (the circulating cooling water is kept at a constant temperature of 5°C); after 2 hours of stable operation of the reaction system Start timing and collect condensed products, stop feeding cyclohexane and NO to the reactor after 24 hours of continuous operation 2 ; After the reaction system is cooled to normal temperature, the product sticking to the outlet of the reactor and the wall of the condenser t...
Embodiment 2
[0103] Embodiment 2: reaction step is the same as example 1, and difference is, also pass into O simultaneously in the reaction process 2 , making cyclohexane:NO 2 :O 2 The molar ratio is 0.8:1:0.1. According to the analysis result and material balance to whole liquid phase and solid phase product, the transformation rate of cyclohexane is 35.7%, and the selectivity of adipic acid and nitrocyclohexane is respectively 50.2% and 48.4% (both total selection sex is 98.6%). Finally, nitrocyclohexane with a purity of 98.4% and adipic acid with a purity of 99.5% were obtained by separating and purifying the liquid and solid phases.
Embodiment 3
[0104] Embodiment 3: The operating steps are the same as in Example 1, except that no catalyst is used. According to the analysis result and material balance to whole liquid phase and solid phase product, the transformation efficiency of cyclohexane is 9.7%, and the selectivity of adipic acid and nitrocyclohexane is respectively 34.1% and 58.4% (both total selections sex is 92.5%). Finally, nitrocyclohexane with a purity of 98.2% and adipic acid with a purity of 99.6% were obtained by separating and purifying the liquid and solid phases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com