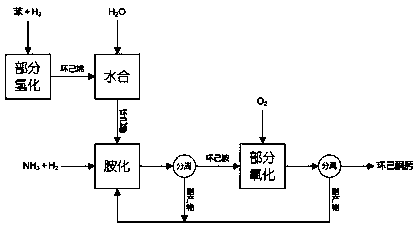
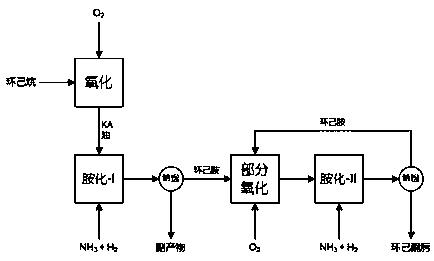
Preparation method of cyclohexanone-oxime
A technology of cyclohexanone oxime and cyclohexanone, which is applied in the field of preparation of cyclohexanone oxime, can solve the problems of high difficulty, high consumption of cyclohexane, large waste lye treatment load, etc., and achieve reduction of material consumption and energy consumption, Reduction of land occupation and investment, the effect of land occupation and investment reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of cobalt-cerium composite oxide catalyst: Weigh 4.4g of cerium nitrate hexahydrate and 1.5g of cobalt nitrate hexahydrate respectively, then add 250ml of deionized water, stir to dissolve completely, obtain solution A, add NaBH at room temperature 4 , to partially reduce the cobalt ions and cerium ions in the A solution. Then weigh 3.1 g of potassium carbonate and place it in a 100 ml beaker, add 50 ml of deionized water and stir to completely dissolve the potassium carbonate to obtain solution B. Then slowly add solution B to solution A under ultrasonic conditions, carry out co-precipitation reaction at 40°C, then add ammonia water dropwise to the mixture containing the precipitate under stirring conditions to adjust the pH value of the solution, and keep stirring to make the pH of the solution The value was stable at 10, and the obtained precipitate was filtered, washed, dried at 120°C for 12 hours, and then calcined in a muffle furnace at 400°C for 2 hou...
Embodiment 2
[0060] Amination of KA oil with ammonia and hydrogen: In a fixed-bed device, load a 6 cm high (1.2 g) amination catalyst NiCu / MgAlO, feed the KA oil obtained in Example 1, at 170 ° C, in normal Under pressure condition, pass into ammonia and hydrogen to carry out ammoniation reaction, wherein KA oil feeding liquid volume space velocity is 0.3h-1, the feeding speed of ammonia is 50mL / min, ammonia and KA oil molar ratio is 13: 1. The hydrogen feed rate is 10mL / min, the reaction product is condensed, and the product is collected every 6 hours for gas chromatography analysis. The conversion rate of cyclohexanol is 98.8%, the conversion rate of cyclohexanone is 100%, and the conversion rate of cyclohexanone is 100%. The selectivity of the amine was 98.5%, and the catalyst activity did not decrease after 200 hours of reaction.
Embodiment 3
[0062] Partial oxidation of cyclohexylamine: take by weighing 15 grams of cyclohexylamine (purity is 99.9%) and 0.5 gram of TiO produced by the method described in Example 2 2 / MCM-41 catalyst were added together into a 100ml reaction kettle, and oxygen was introduced (the pressure was maintained at 1.0 MPa), and the reaction was carried out at 100°C for 4 hours. After the reaction, the solid catalyst was separated by filtration to obtain 16.25 grams of oxidation reaction liquid. This solution was qualitatively characterized by a gas spectrometer, and accurately quantified by a gas chromatography internal standard method (chlorobenzene was used as an internal standard). The measured cyclohexylamine was 8.78 grams, cyclohexanone oxime was 6.42 grams, and the by-product nitrate 0.45 gram of cyclohexane, 0.15 gram of cyclohexanone, 0.07 gram of cyclohexyl imine, 0.03 gram of N-cyclohexyl cyclohexyl imine, the conversion rate of cyclohexylamine in this process is 41.6%, the selecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com