Fusion protein as well as amino acid sequence, coding nucleotide sequence, preparation method and application thereof
A technology of nucleotide sequence and fusion protein, applied in botanical equipment and methods, applications, fusion polypeptides, etc., can solve the problems of difficult to make scientific and effective guidance, high concentration of use, unclear receptors, etc., to achieve Reduce the ability of pathogenic microorganisms to infect, use low concentration, and reduce the effect of agricultural production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 7
[0145] Example 1 Molecular Design and Nucleotide Sequence Acquisition of Heptapeptide Fusion Protein His-MP7
[0146] (1) Among 11 different PAMP molecular polypeptides: flg22, nlp20, elf18, pip1, pep1, csp22, flgII-28, elf18, pep13, ralf17, hrp15, sys18 and their mutants, select 7 different PAMP molecules Polypeptides: flg22, nlp20, elf18, pip1, pep1, csp22, flgII-28 form a fusion protein, and the linkers are AGA and GAG;
[0147] (2) Use the online analysis platform ExPASy (https: / / www.expasy.org) to conduct basic property analysis (protscale) such as molecular mass, amino acid composition (protparam) and hydrophobicity of the above protein sequence; use Phyre2 online platform (http : / / www.sbg.bio.ic.ac.uk / phyre2 / html / page.cgi?id=index) to model the above protein structure;
[0148] (3) Based on the analysis results, one design scheme was screened out, and the fusion protein was named MP7, with the amino acid sequence shown in SEQID NO:15;
[0149] (4) Utilize bioinformati...
Embodiment 2 7
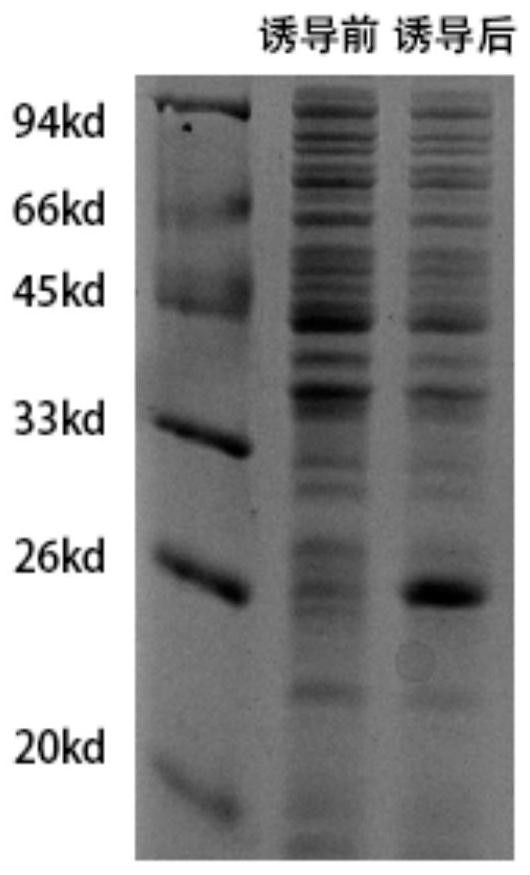
[0150] Example 2 Expression and purification of the heptapeptide fusion protein His-MP7
[0151] (1) Cloning the nucleotide sequence shown in SEQ ID NO:16 into the HindIII and XholI sites of the pET-28b (+) expression vector (Novagen), heat-shocked transformation into Escherichia coli DH5α, and picking positive clones, Shake the bacteria and extract the plasmid, after enzyme digestion and sequencing to verify correctness, transform into Escherichia coli BL21(DE3) by heat shock, and the Escherichia coli containing the recombinant plasmid pET-28b-MP7 is named BL21(DE3) / pET-28b-MP7 ;
[0152] (2) Inducing the expression of BL21(DE3) / pET-28b-MP7, including the following steps: the expression strain was inoculated in LB liquid medium, 37 ° C, 200 rpm / min shaking culture overnight, to obtain the first bacterial liquid; overnight The bacterial solution was transferred to LB liquid medium containing 100 μg / mL kanamycin at a volume ratio of 1:100, and continued to shake at 37°C and 20...
Embodiment 3 7
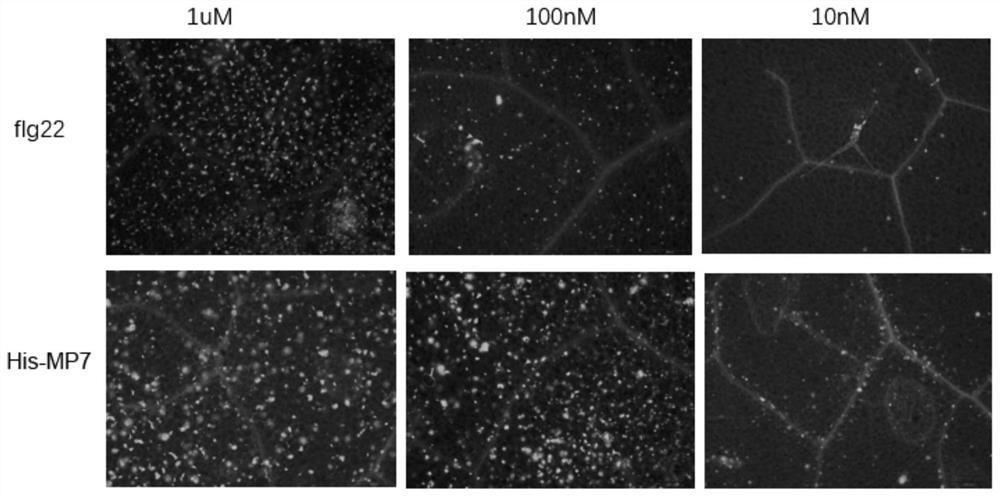
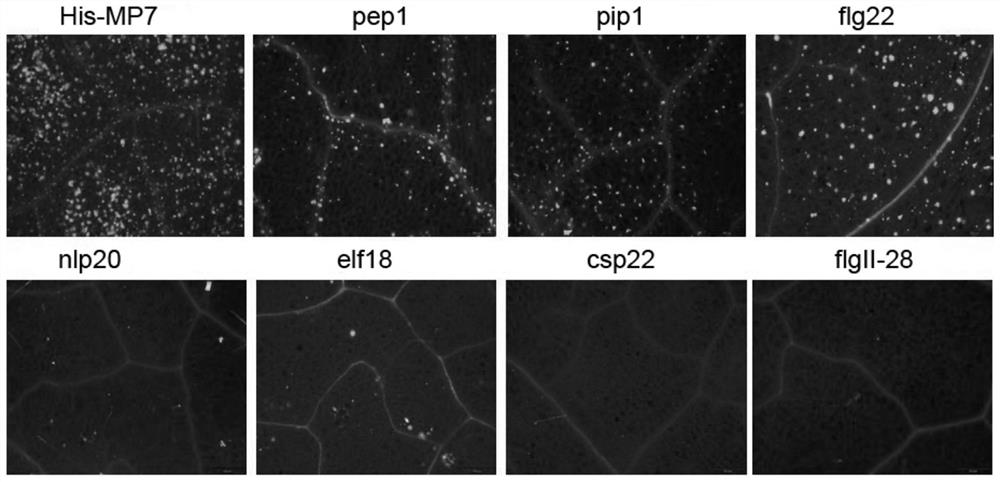
[0155] Example 3 Study on the Potency of Immune Activation of Heptapeptide Fusion Protein His-MP7
[0156]The accumulation of callose will occur in the immune response of plant cells. Callose can strengthen the mechanical strength of plant cell walls and block the channels for pathogens to spread between cells, thereby limiting the invasion of pathogenic microorganisms. Using the model plant Arabidopsis thaliana as the material and callose accumulation as the immune indicator, the immune activation ability of the fusion protein His-MP7 at different concentrations was analyzed, and compared with a single PAMP molecule polypeptide. The specific experimental operation is as follows:
[0157] 1. Comparison of callose accumulation in Arabidopsis thaliana caused by different concentrations of fusion protein His-MP7
[0158] Adjust the final concentrations of the fusion protein His-MP7 and polypeptide flg22 solutions to 1 μM, 100 nM, and 10 nM, respectively, and use water as a bla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com