Palladium catalyst for catalyzing quinazolinone synthesis and olefination reaction
A palladium catalyst, oxidation reaction technology, applied in the direction of carbon compound catalyst, catalytic reaction, organic compound/hydride/coordination complex catalyst, etc. The effect of excellent catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Preparation of Homogeneous Palladium Catalyst:
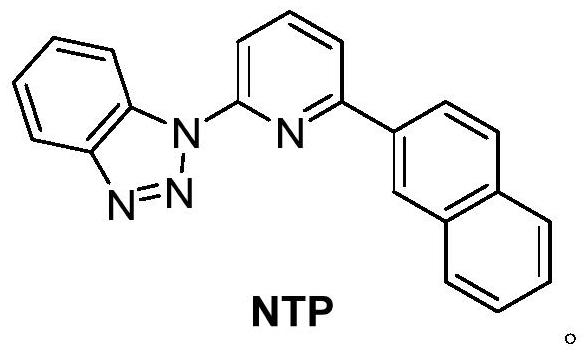
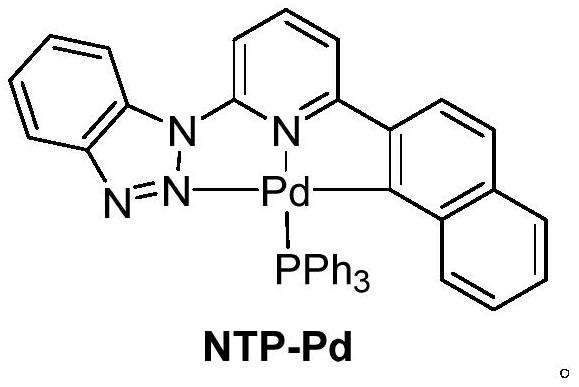
[0071] Under nitrogen atmosphere, the PdCl 2 (PPh 3 ) 3 (192 mg, 0.2 mmol), a mixture of L (64 mg, 0.2 mmol) and triethylamine (126 mg, 1.25 mmol) in isopropanol (5.0 mL) was refluxed for 8 hours. The mixture was cooled to room temperature to precipitate a reddish-brown microcrystalline solid. The solid was filtered off, washed with ether (3 x 15 mL), and dried under vacuum to give the homogeneous palladium catalyst NTP-Pd.
Embodiment 2
[0072] Example 2: Preparation of Heterogeneous Palladium Catalyst:
[0073] Cetyltrimethylammonium bromide (CTAB, 0.3 g) was dissolved in water (35.6 mL), potassium hydroxide (4.7 mL, 2 mol / L) was added, and the mixture was stirred at room temperature for 30 minutes. Then butyl orthosilicate (TBOS) was added dropwise, then stirred at room temperature for 13 hours, then aged for 12 hours, and filtered to obtain a white solid, which was then washed three times with water and then three times with ethanol. Finally, it was dried at a high temperature of 600 °C for 12 h to obtain mesoporous silica MSM.
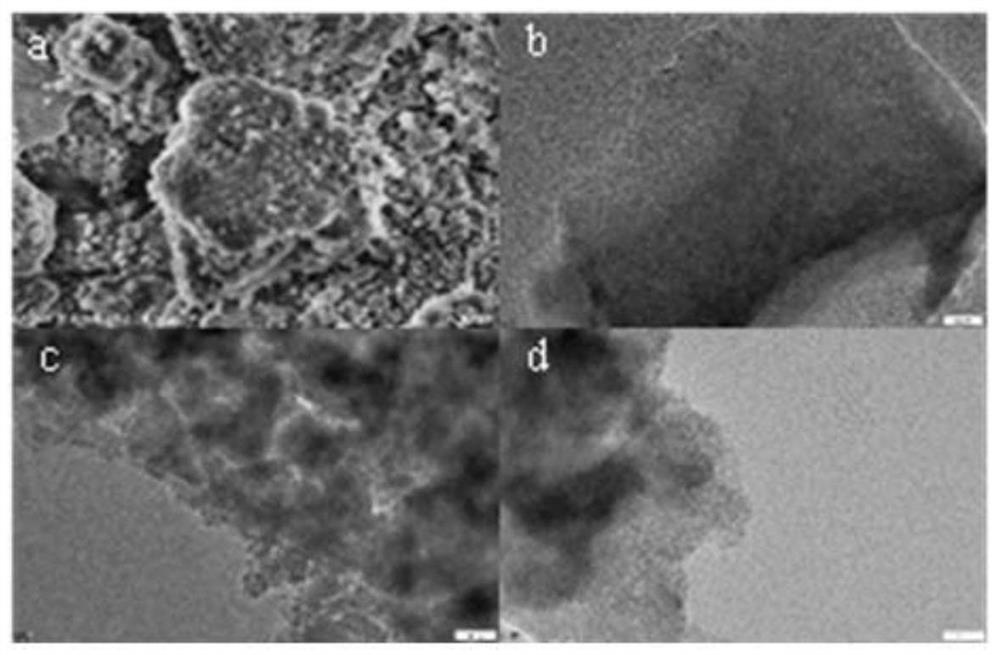
[0074] Mesoporous silica MSM (1.0 g) and APTES (1.0 mL) were refluxed in toluene (100 mL) for 24 hours under nitrogen atmosphere. The mixture was cooled to room temperature by centrifugation to obtain a solid, which was then washed 3 times with ethanol. Finally, the modified MSM (450 mg), palladium complex (10 mg) and ethanol (4 mL), toluene (10 mL) solutions were combined under ...
Embodiment 3
[0077] A novel homogeneous palladium-catalyzed quinazolinone series reaction, comprising the following processing steps:
[0078] Under nitrogen atmosphere, in a 25 mL Schlenk flask, mix 2-aminobenzonitrile (0.5 mmol), benzyl alcohol (0.5 mmol) NTP-Pd catalyst (1.0 mol%), Cs 2 CO 3 (0.75 mmol) were sequentially introduced into a 25 mL Schlenk tube. Then the solvent DMSO:H is added 2 O (2.0 ml, 2:1), stirred at 120°C for 12 hours. After cooling to room temperature, water was added to quench the reaction and extracted with ethyl acetate, the organic phase was concentrated by removing the solvent in vacuo. Finally, the residue was purified by column chromatography using petroleum ether as eluent to give the desired product. The yield was 82%.
[0079] Product structure:
[0080] Structure Characterization:1 H NMR (400MHz, DMSO) δ 12.54 (s, 1H), 8.18 (dd, J=9.8, 8.2Hz, 3H), 7.91–7.80 (m, 1H), 7.75 (d, J=8.0Hz, 1H) ,7.64–7.47(m,4H). 13 C NMR (101MHz, DMSO) δ162.76, 152.84...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com