A kind of echinococcus multilocularis glucose transporter polypeptide vaccine glep and its preparation method and application
A technology of glucose transport and Echinococcus multilocularis, applied in the field of application and preparation of Echinococcus multilocularis glucose transporter polypeptide vaccine GLEP, can solve the problem of drug resistance, large side effects, and poor therapeutic effect of human echinococcosis and other issues to achieve the effect of strong stability, high safety and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Construction of recombinant expression vector pCzn1-GLEP (containing fusion gene GLEP)
[0041] The amino acid sequence of GLEP was converted into the corresponding nucleotide sequence according to the codon preference principle of Escherichia coli, and the full-length splicing primer was designed based on the PAS (PCR-based Accurate Synthesis) method, and protection was designed at both ends of the primer. The sex base synthesis gene GLEP was connected into the expression vector pCzn1 through the cloning sites Nde I and Xba I.
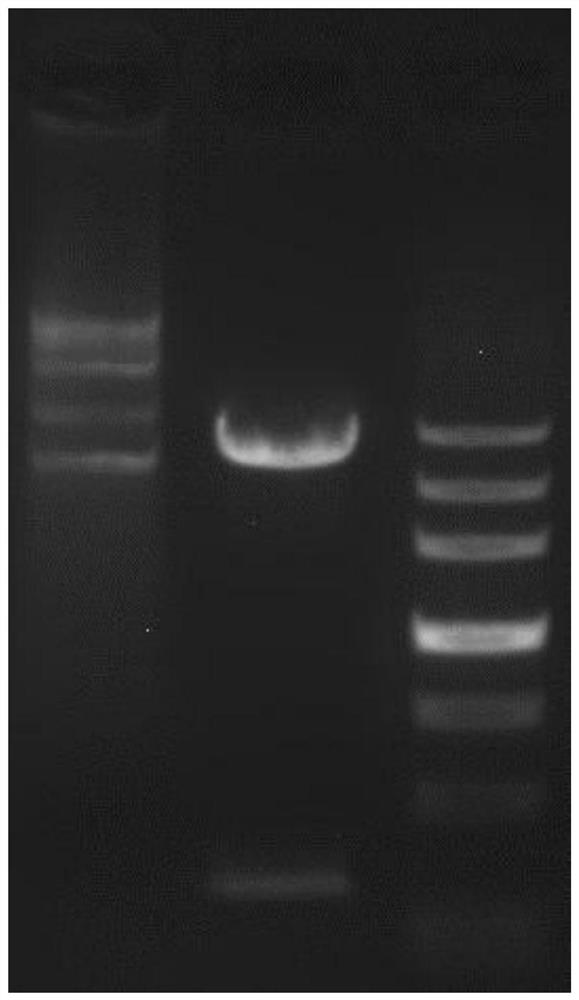
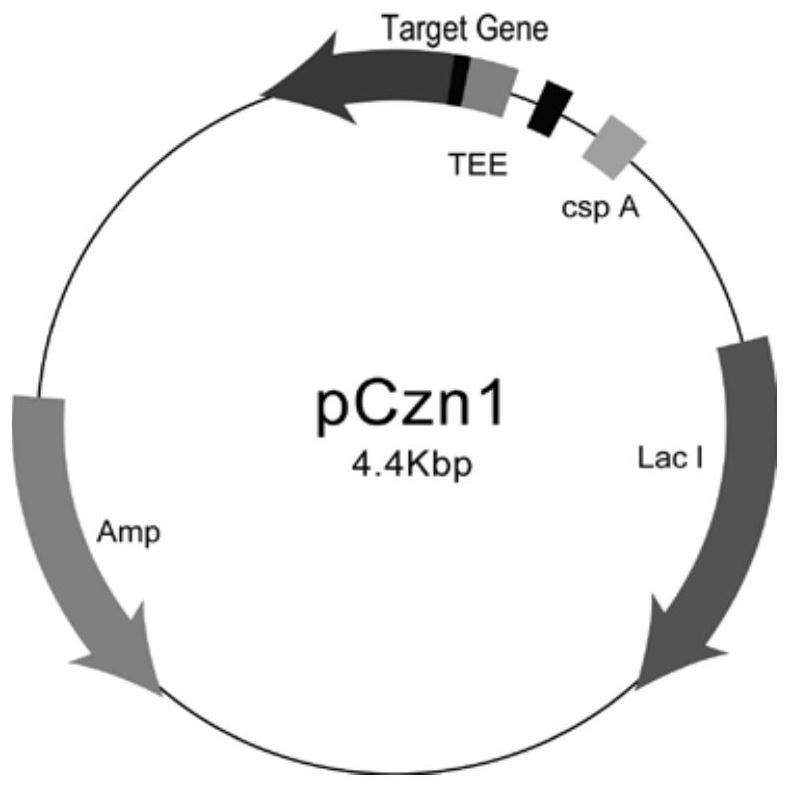
[0042] Results: The recombinant plasmid pCzn1-GLEP to be detected was digested with Nde I and Xba I, reacted at 37°C for 2 hours, and detected by 1% agarose gel electrophoresis. The theoretical size of the same, such as figure 1 shown. The vector construction map of the recombinant expression vector pCzn1-GLEP is as follows: figure 2 shown. The obtained recombinant plasmid pCzn1-GLEP was transformed into the TOP10 clone strai...
Embodiment 2
[0043] Embodiment 2: the prokaryotic expression of polypeptide fusion protein GLEP
[0044] The correct recombinant expression plasmid pCzn1-GLEP was verified to be transferred into Escherichia coli Arctic Express strain. On the pre-prepared LB plate containing 50 μg / mL Amp, inoculate the loop-streaked genetically engineered strain pCzn1-GLEP / ArcticExpress, place it upside down in a 37°C incubator, and after culturing overnight, pick a single colony and inoculate it on a plate containing 50 μg / mL In the LB medium of Amp, cultivate overnight at 37°C, 220rpm. Inoculate the recombinant bacteria with 2% inoculation amount in LB medium containing 50 μg / mL Amp, 37 ° C, 220 rpm, cultivate until the OD600 of the bacteria is 0.6-0.8 (about 2 hours), add IPTG to make the final concentration reach 1 mmol / L, The expression was induced at 37°C and 220rpm for 4 hours, and the carrier strain pCzn1-GLEP / Arctic Express induced without IPTG was used as a negative control.
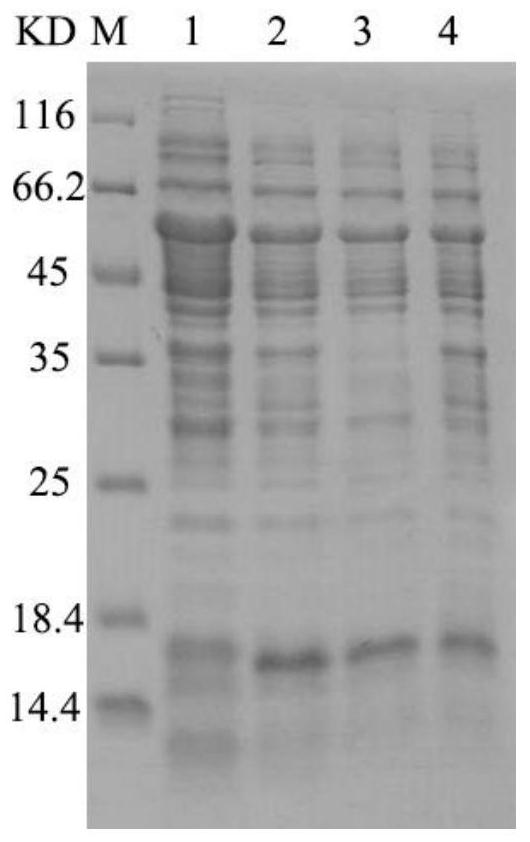
[0045] Results: Co...
Embodiment 3
[0046] Embodiment 3: Purification of polypeptide fusion protein GLEP
[0047] (1) Cell disruption of recombinant bacteria
[0048] Resuspend the cell pellet in 20ml lysis buffer (20mM Tris-HCl containing 1mM PMSFand bacteria protease inhibitor cocktail, pH 8.0), ultrasonically break (power 400W, work 4sec, intermittent 8sec, total 20min); the ultrasonically broken cell lysate Centrifuge at 10,000 g for 20 min at 4°C and collect the supernatant;
[0049] (2) Purification of Ni-IDA nickel ion affinity chromatography column
[0050] Using the low-pressure chromatography system, the protein solution was loaded onto the Ni-IDA-Sepharose CL-6B affinity chromatography column pre-equilibrated with Ni-IDA Binding-Buffer at a flow rate of 0.5ml / min; Wash at a flow rate of 1mL / min until the OD280 value of the effluent reaches the baseline; wash with Ni-IDA Washing-Buffer (20mM Tris-HCl, 20mM imidazole, 0.15M NaCl, pH8.0) at a flow rate of 1mL / min until the OD280 value of the effluent r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com