Recombinant bacterium capable of producing L-homoserine at high yield as well as preparation method and application of recombinant bacterium
A technology of homoserine and recombinant bacteria, applied in the biological field, can solve the problems of unfriendly environment, lack of homoserine, and high cost, and achieve the effect of realizing reducing power balance and enhancing glyoxylic acid cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1, inventive thinking
[0080] The dual-channel 1:1 ratio is used to achieve cofactor balance, as follows:
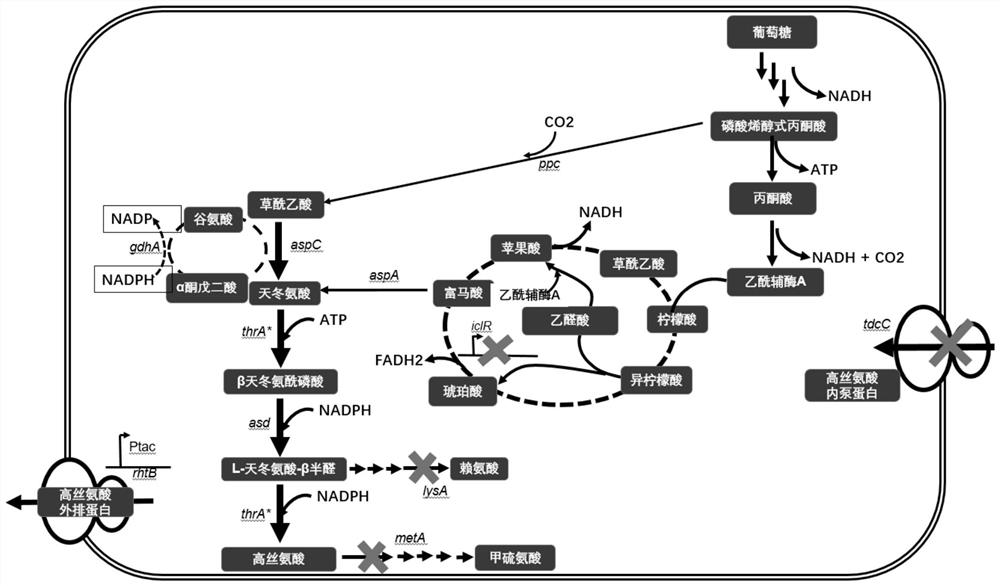
[0081] Cofactor balance is a very important factor in the process of metabolic pathway modification. The imbalance of cofactors will cause the disadvantages of slow cell growth and low product yield. In the present invention, taking the metabolic pathways of homoserine and threonine as an example, there are two pathways in Escherichia coli to generate L-homoserine and L-threonine. The first is the conventional oxaloacetate synthesis aspartic acid route, under anaerobic conditions, the theoretical conversion ratio from glucose to product is 1:2, but it lacks the reducing power of 4 NADPH: 1 Glucose+6 NADPH +2 CO2+2ATP=2 Homoserine / Threonine+2 NADH; the second way is to knock out the glyoxylate cycle inhibitor gene iclR, enhance the glyoxylate cycle of E. Express the aspA gene, enhance the synthesis ability of fumaric acid to aspartic acid, the theore...
Embodiment 2
[0082] Example 2, Construction of chassis strains MG1655 (△lacI, △thrB::Trc-ppc, △metA, △lysA, △sthA::Trc-pntAB)
[0083] Using Crispr / cas9 technology to knock out, replace or insert genes on the MG1655 chromosome, the construction of the chassis strain mainly includes: knocking out the threonine metabolism pathway homoserine kinase thrB, knocking out the methionine metabolism bypass homoserine succinyltransferase metA, Knockout of lysine metabolism bypass diaminoheptanoate decarboxylase lysA, knockout of glyoxylate pathway inhibitory protein iclR, overexpression of phosphoenolpyruvate carboxylase ppc, knockout of soluble pyridine nucleotidyl transferase sthA, Overexpression of the pyridine nucleotidyl transferase pntAB, as follows:
[0084] 1. Knockout of the DNA-binding transcriptional repressor lacI (NP_414879.3, submission date 20181011)
[0085] 1. Construction of MG1655 / pCAS
[0086] The pCAS plasmid (kanamycin resistance) was introduced into MG1655. Because the pCAS p...
Embodiment 3
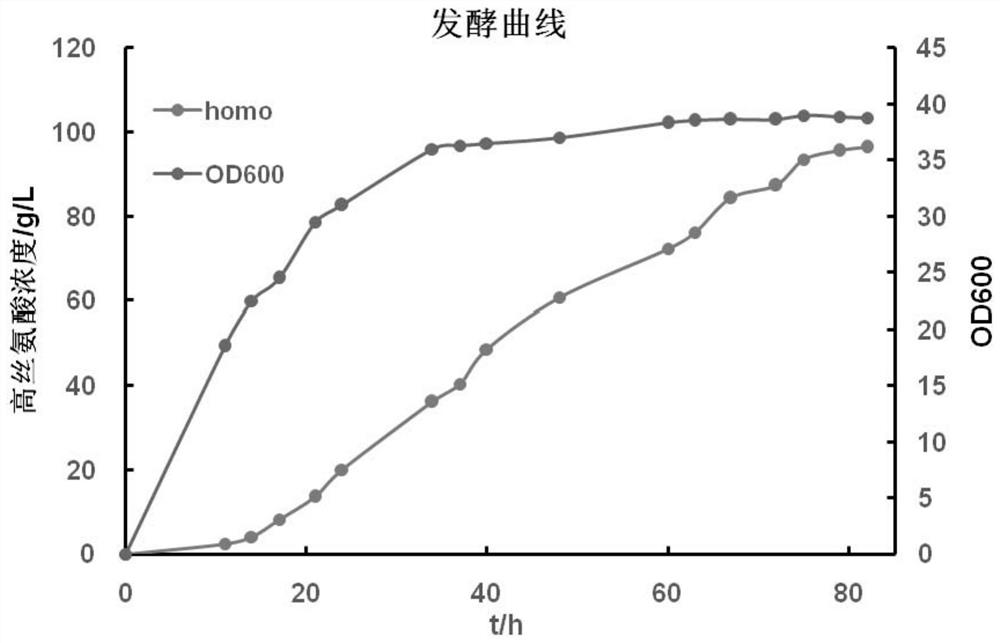
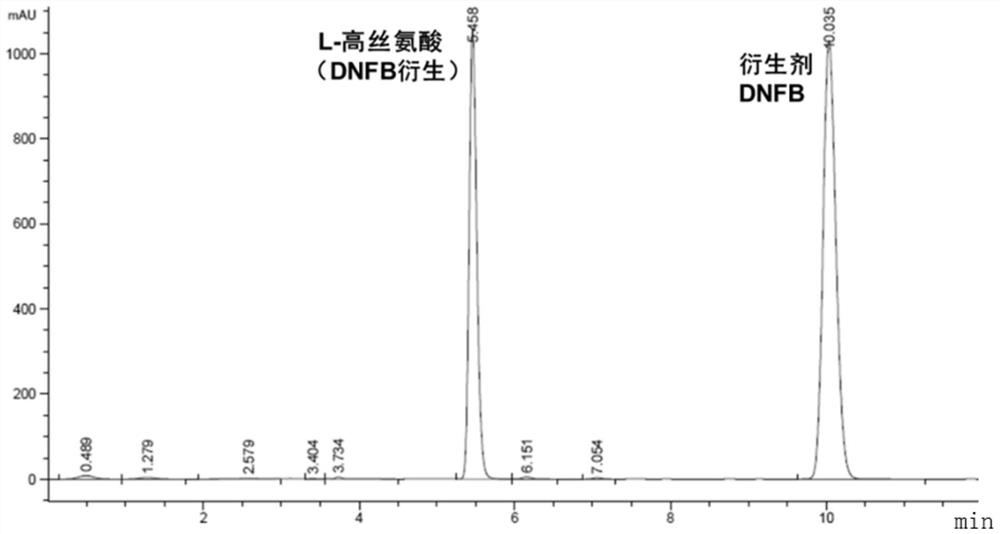
[0129] Example 3, Preparation of recombinant bacteria overexpressing thrA* and asd and its application in improving homoserine production
[0130] 1. Preparation of recombinant bacteria overexpressing thrA* and asd
[0131] The commercial plasmid pTRC99A was used to overexpress aspartokinase I mutant thrA* (1034C-T) and aspartate semialdehyde dehydrogenase asd to increase the flux from aspartic acid to homoserine; the specific method is as follows:
[0132] pTRC99A was double digested with NcoI and HindIII, and the 4120bp pTRC99A digestion product was collected.
[0133] Using MG165 genomic DNA as a template, amplify respectively with primers thrA-F / R and asd-F / R (Table 2), obtain the thrA gene of 2463bp (the nucleotide sequence is compared with sequence 8, only the 1034 base C, other bases are the same) and the 1104bp asd gene (SEQ ID NO: 9).
[0134] The above-mentioned 4120bp pTRC99A digestion product, 2463bp thrA gene and 1104bp asd gene were ligated using Gibson seamles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com