Aspartase mutant with improved enzyme activity and changed optimal pH
An aspartase and aspartic acid technology, applied in the field of genetic engineering, can solve the problems of harsh pH value conditions, low enzyme activity, and high production cost of β-amino acids, and achieves enhanced capacity and improved specific enzyme activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Contain the recombinant expression vector of the gene encoding aspartase mutant and the construction of recombinant bacteria
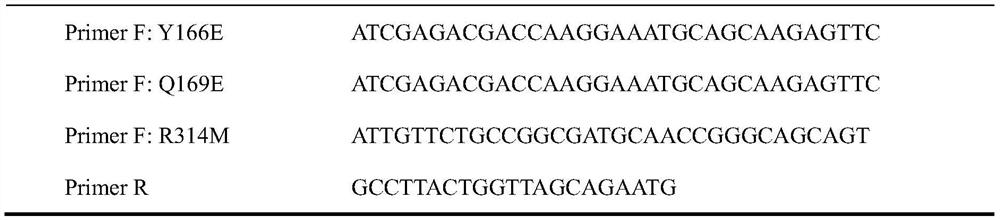
[0028] Mutate leucine at position 12 to aspartic acid, tyrosine at position 166 to glutamic acid, glutamine at position 169 to glutamic acid, arginine at position 314 to formazan Thionine, the specific method is: with the pET-21a recombinant plasmid (restriction sites EcoR I and BamH I) containing the nucleotide sequence shown in SEQ ID NO.4 as template, the sequence shown in Table 1 is primer, using The mutant plasmid was constructed by the two-step PCR method of the whole plasmid (the reaction system is shown in Table 2, and the reaction conditions are shown in Table 3), and the gene shown in SEQ ID NO.2 was obtained.
[0029] Table 2 PCR reaction system
[0030]
[0031] Table 3 PCR reaction conditions
[0032]
[0033] The PCR product was checked by gel electrophoresis, and then 1 μL of Dpn I restriction endonuclease wa...
Embodiment 2
[0034] Example 2: Expression of recombinant bacteria pET21a-L12D-Y166E-Q169E-R314M / E.coli BL21
[0035] The recombinant strain pET21a-L12D-Y166E-Q169E-R314M / E.coli BL21 constructed in Example 1 and the control strain pET21a- AspB / E.coli BL21 were inoculated in 10 mL of LB medium containing ampicillin, cultured with shaking at 37°C overnight, until OD 600 0.6-0.9 The next day, transfer to 50mL LB medium containing ampicillin according to 1% inoculation amount, culture at 37°C for 2-3h, then add 0.5mM IPTG and induce at 16°C for 12-16h. The cells were collected by centrifugation at 8000 rpm for 10 min at 4° C. and crushed, and the cell crushed supernatant (crude enzyme liquid) was collected for subsequent purification.
[0036] Purification of aspartase or aspartase mutants is carried out in a hot water bath at 60° C. for 30 minutes, and then centrifuged at 12000 rpm for 90 minutes to obtain the purified enzyme. The obtained purified enzyme was stored at 4°C until use. The pu...
Embodiment 3
[0037] Example 3: Aspartase activity assay and HPLC detection of β-aminobutyric acid
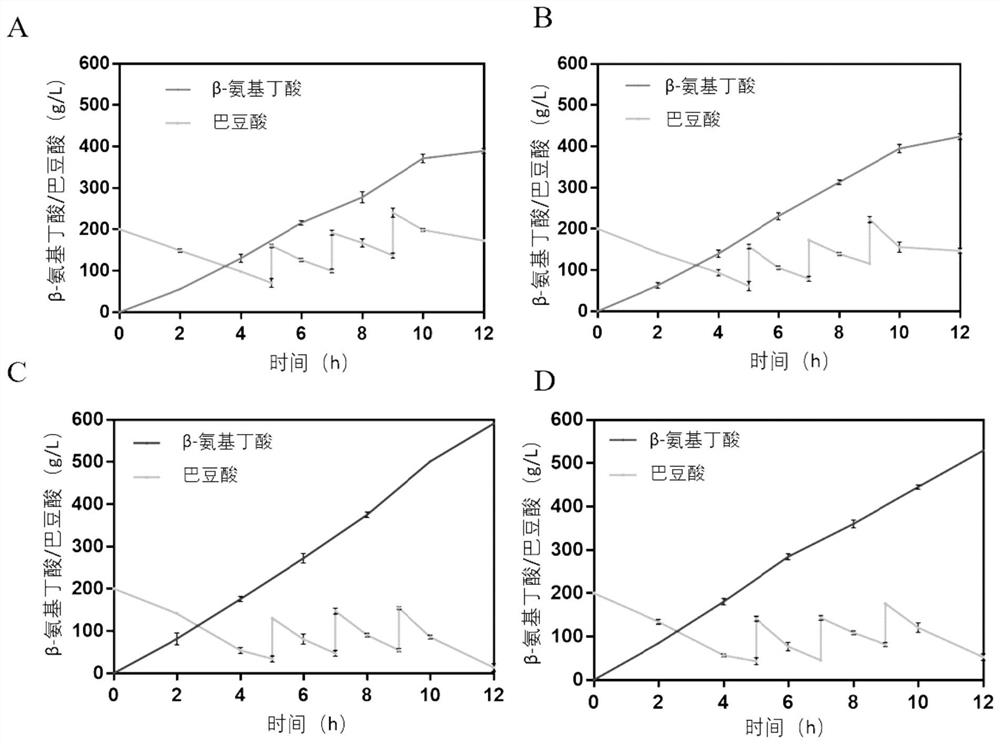
[0038] The enzyme activity of the original enzyme and the mutant enzyme was measured at 70°C and pH 7.0, 8.0, 9.0, and 10.0. After reacting for 1 hour, the content of the product β-aminobutyric acid was measured by HPLC.
[0039] HPLC: Take 100 μL reaction solution, add 40 μL 1M NaHCO 3 After mixing, add 160 μL of 2,4-dinitrofluorobenzene (20.48 mg dissolved in 3 mL of acetone), react in the dark at 60 ° C for 1 h, take it out and centrifuge, and filter with a 0.22 μ membrane. Then inject. Chromatographic column: dimosoil C18 (5μL, 250mm×4.6mm), mobile phase: A: 0.1% formic acid aqueous solution, B: 100% acetonitrile, detector: UVDetector, detection wavelength: 360nm, column temperature: 25°C, injection volume: 10 μL, flow rate: 1.0 mL / min. Process: 0~22min: 15%B→50%B; 22~22.1min: 50%B→15%B; 22.1~26min: 15%B.
[0040] The calculated specific enzyme activities of the original enzyme and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com