Process method for preparing succinic acid glycol ester
A technology of diol succinate and a process method, applied in the field of preparing diol succinate, can solve the problems of infeasible industrial production, violation of chemical thermodynamic principles, impossible to realize and maintain, etc., to reduce energy consumption, The effect of improving the total yield and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
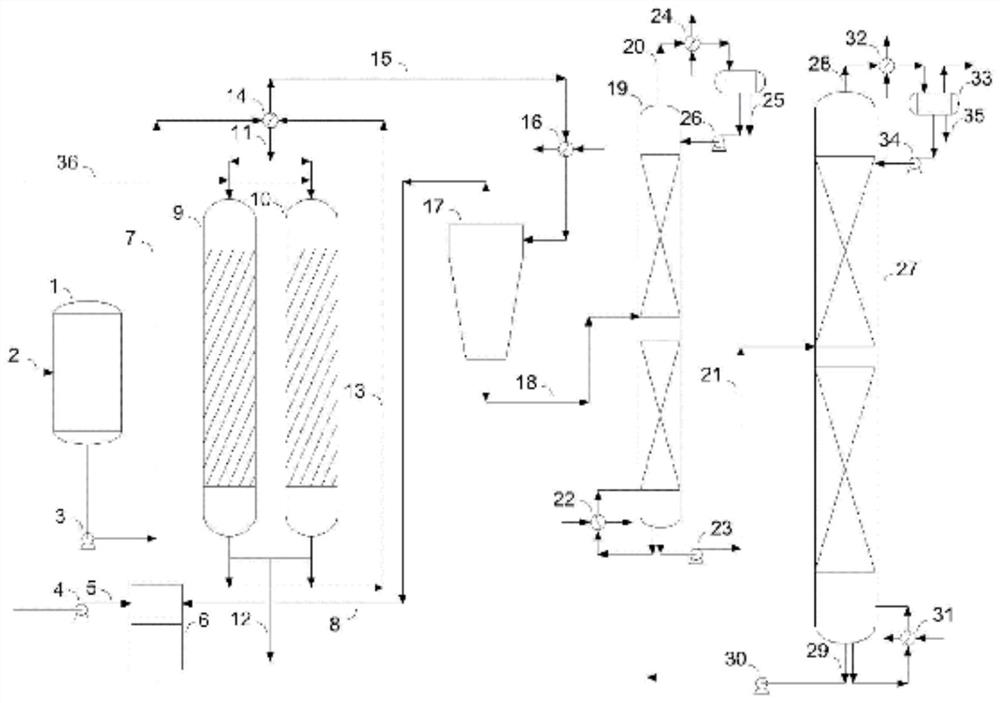
Image
Examples
Embodiment 1
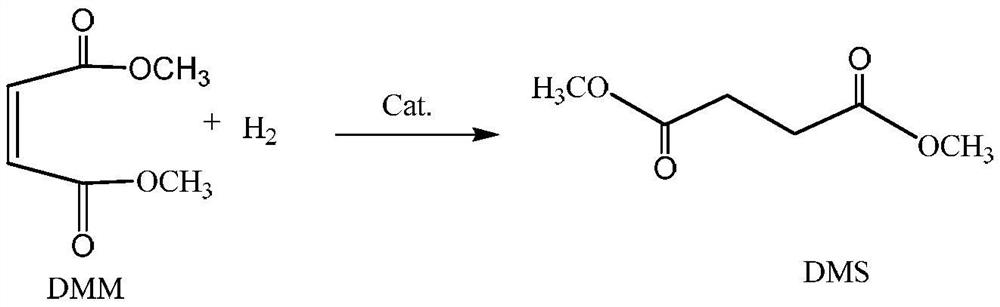
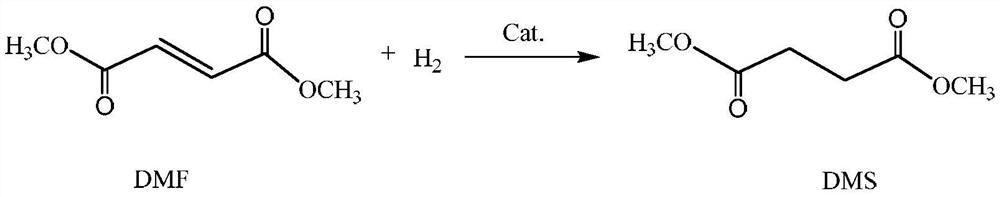
[0048] According to the method provided by the applicant of this patent in the original application "A kind of process for preparing diol maleate" (application number is 202110180196.7), using maleic anhydride and methanol as raw materials, synthesize 1500g of dimethyl maleate Ester DMM is the main component of crude DMM-1, and its composition is shown in Table 1.
[0049] Table 1: Composition (wt%) of crude DMM-1
[0050]
[0051] Selective hydrogenation of crude DMM-1 was carried out on a self-made fixed bed with a catalyst of 0.3% Pd / Al 2 o 3 The supported catalyst, the carrier active Al 2 o 3 Modified with 0.3% alkali metal K; the loading amount is 6.0g, and it is filled in an isothermal reaction tube reactor using Φ12.7X1.6 (SS316). The temperature is measured by a sleeve-type thermocouple inside and a feedback resistor is used outside Furnace heating and temperature control. The liquid crude DMM-1 is fed by the Yilite P230II high-pressure liquid metering pump, an...
Embodiment 2
[0056] Also according to the method of Example 1, maleic anhydride and methanol were used as raw materials to synthesize 4500 g of crude DMM-2 with dimethyl maleate DMM as the main component, and its composition is shown in Table 3.
[0057] Table 3: Composition (wt%) of crude DMM-2
[0058]
[0059] In this example, the reaction device and catalyst loading for selective hydrogenation of crude DMM-2 are the same as in Example 1. In the reaction conditions, the molar ratio of crude DMM-2 to hydrogen is 1:20, the pressure is 3.1MPa, and the crude DMM-2 The mass space velocity of 2 is 2.0h -1 . When the reaction temperature reaches a steady state at 220° C., the conversion rate of DMM in the reaction is 99.75%, and the selectivity of DMS is 99.87%. The composition of the reaction product is shown in Table 4 Composition-2. When the reaction temperature reaches a steady state at 240° C., the conversion rate of DMM in the reaction is 100%, and the selectivity of DMS is 99.80%. ...
Embodiment 3
[0064] The reaction raw material selected in this embodiment is the thick DMM-2 used in embodiment 2, the reaction device of selective hydrogenation and the catalyst loading are the same as in Example 1, and the mol ratio of thick DMM-2 and hydrogen in the reaction conditions is 1:20, the pressure is 3.1MPa. When the mass space velocity of crude DMM-2 is 3.0h -1 When the reaction temperature reaches a steady state at 240° C., the conversion rate of DMM in the reaction is 100%, and the selectivity of DMS is 100.47%. The composition of the reaction product is shown in Table 5 Composition-4. When the mass space velocity of crude DMM-2 is 4.0h -1 When the reaction temperature reaches a steady state at 250° C., the conversion rate of DMM in the reaction is 100%, and the selectivity of DMS is 99.81%. The composition of the reaction product is shown in Table 5 Composition-5. When the mass space velocity of crude DMM-2 is 6.0h -1 When the reaction temperature reaches a steady state...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com