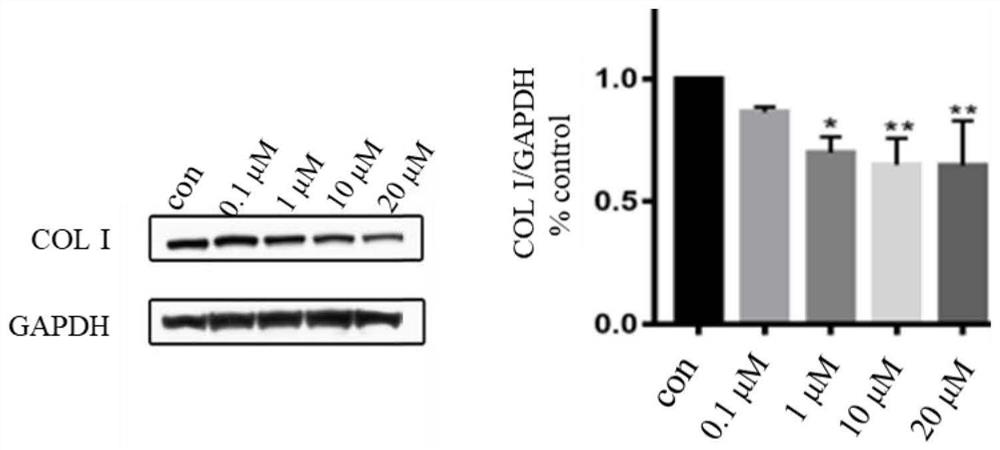
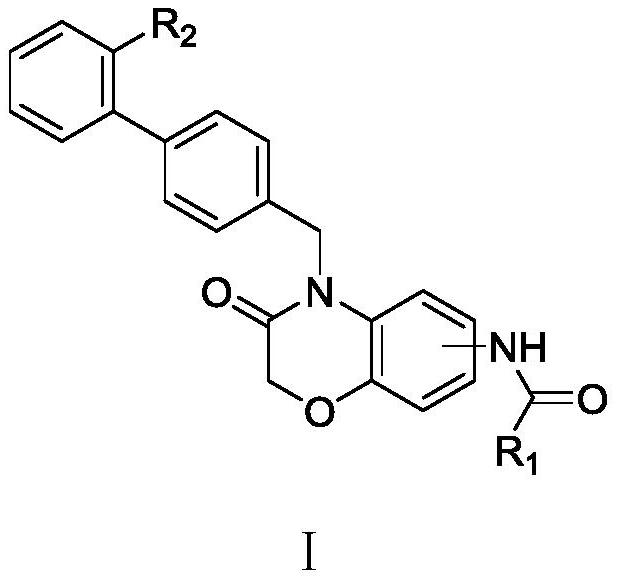
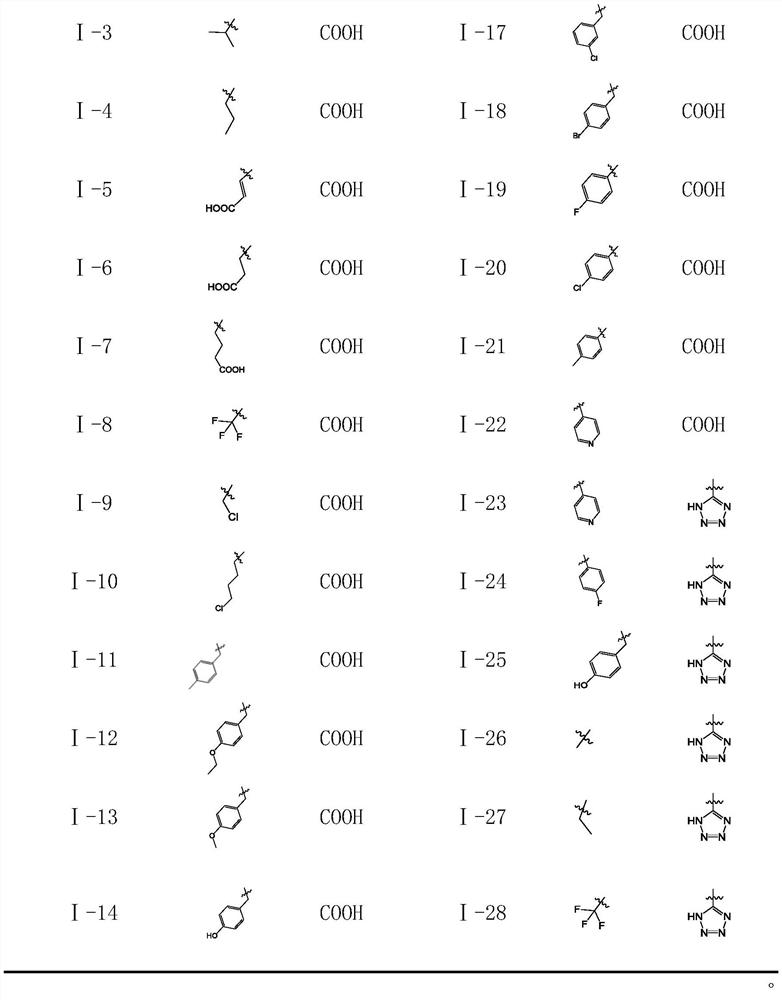
Compound containing benzomorpholone-biphenyl skeleton as well as preparation method and application thereof
A technology of benzomorpholone and skeleton compounds, which is applied in the field of medicinal chemistry, can solve the problems of lack of oral activity and low selectivity, and achieve the effects of simple synthesis method, vasodilation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the preparation of compound I-1
[0041] 2-Hydroxy-4-nitroaniline (0.50g, 3.25mmol), sodium carbonate (1.09g, 12.98mmol), benzyltriethylammonium chloride (0.74g, 3.25mmol) were dissolved in 20mL ethanol, in Under the condition of ice bath, slowly add the mixture of chloroacetyl chloride and ethanol dropwise, and continue to stir for 40min after dropping. Subsequently, the reaction bottle was moved to an oil bath, and after reflux reaction for 2 h, the reaction was complete as monitored by TLC. The solid matter in the reaction system was removed by filtration while hot, and the residue was recrystallized with ethanol after rotary evaporation to remove the solvent. Intermediate 2 was obtained by suction filtration under reduced pressure, purple needle-like crystals, and the yield was 93%. 1 H NMR (400MHz, DMSO-d 6 )δ11.15(s,1H),7.89(dt,J=8.9,2.7Hz,1H),7.81(d,J=2.7Hz,1H),7.20(dd,J=8.9,2.7Hz,1H), 4.83(s,2H). 13 C NMR (101MHz, DMSO-d 6 )δ164.25, 149.15, 14...
Embodiment 2
[0046] Embodiment 2, the synthesis of compound I-22
[0047] Intermediate 4-1 was prepared in the same manner.
[0048] Add compound 4-1 (300mg, 0.77mmol), isonicotinic acid (0.93mmol), triethylamine (0.93mmol), 1-hydroxybenzotriazole (125.32mg, 0.93mmol), 15mL to a 25mL round bottom flask Anhydrous N,N-dimethylformamide, stirred overnight at room temperature. After the reaction was terminated, 10 mL of water was added to the system, extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and column chromatographed to obtain 5-22, a white solid, with a yield of 90%.
[0049] Compound 5-22 was dissolved in 10 mL of methanol, and then 10 mL of 1 mol / L sodium hydroxide aqueous solution was added dropwise. After heating to reflux for 3 hours, the reaction solution changed from turbid to clear, and TLC detected that the reaction was complete. The methanol was distilled off under reduced pressure, and then 10 mL of water was added ...
Embodiment 3
[0050] Embodiment 3, the synthesis of compound I-24.
[0051] Intermediate 2 was prepared in the same manner.
[0052] Dissolve 2-triphenyltetrazolium-4'-bromomethylbiphenyl (0.91g, 1.64mmol) in 20mL of acetonitrile, then add sodium carbonate (0.28g, 1.97mmol), stir at reflux for 30min, and then add compound 2 (0.32g, 1.64mmol), after the reaction was complete, filtered, the filtrate was concentrated and then separated by column chromatography to obtain the intermediate biphenyl condensation product 3-2: pale yellow solid, the yield was 82%.
[0053] To 20mL ethanol: water (V:V=3:1) mixed solvent, add compound 3-2 (0.81g, 1.20mmol) and zinc powder (0.38g, 5.98mmol), ammonium chloride (0.32g, 5.98mmol ), stirring at reflux for 2h, the reaction was complete. After the reaction solution was filtered while it was hot, the ethanol was removed by rotary evaporation, and then 10 mL of water was added, and the pH was adjusted to 8-9 with aqueous sodium bicarbonate solution. A large ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com