Polyphosphokinase mutants
A polyphosphate kinase and mutant technology, applied in the field of enzyme engineering, can solve the problem that the enzyme activity cannot reach the industrialized large-scale production of glutathione
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Construction of recombinant Escherichia coli expressing wild-type polyphosphate kinase
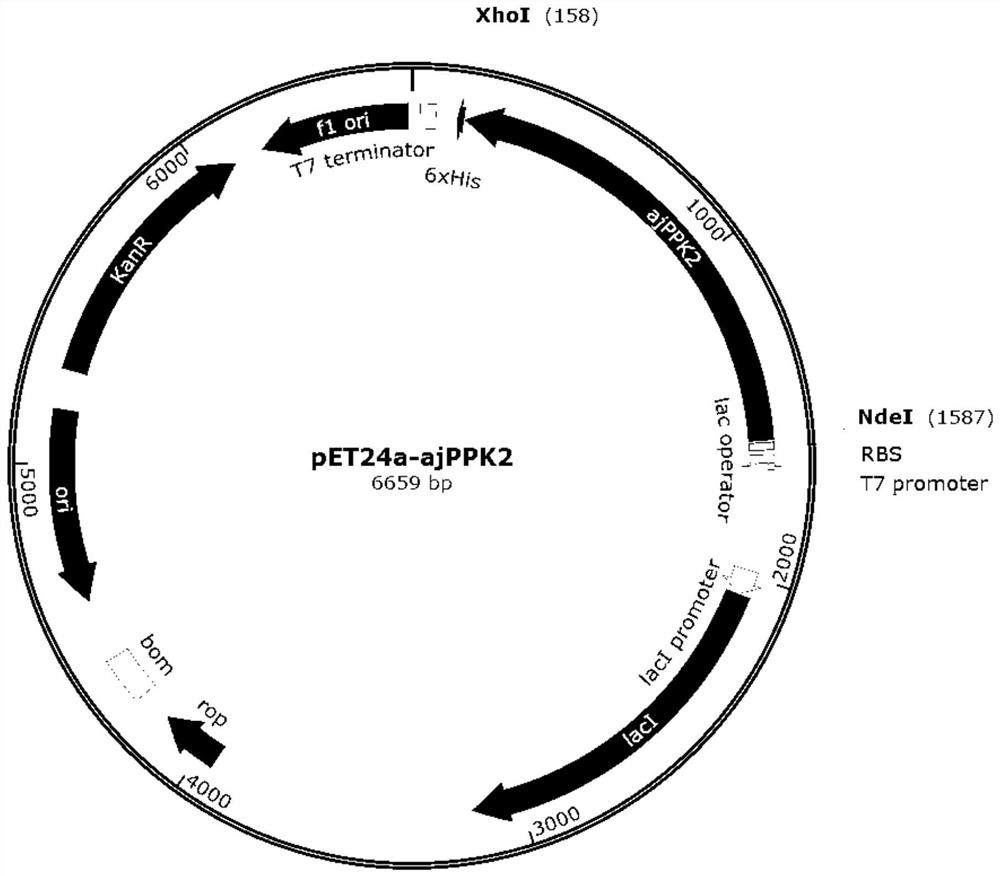
[0040] For the amino acid sequence SEQ ID NO:1 of the polyphosphate kinase (GenBank accession number: AB092983.1) derived from Acinetobacter johnsonii, codon optimization was performed, and the optimized gene sequence was SEQ ID NO:2. The nucleotide sequence of the whole gene is synthesized as SEQ ID NO: 2, and the restriction endonuclease sites XhoI and NdeI are designed at both ends of the gene, and subcloned into the corresponding sites of the vector pET24a (Novagen) to obtain the recombinant plasmid pET24a-ajPPK2 ,like figure 1 shown.
[0041] The recombinant plasmid pET24a-ajPPK2 was electrotransformed into host Escherichia coli BL21(DE3) to obtain recombinant Escherichia coli PET24a-ajPPK2 / BL21(DE3) expressing wild-type polyphosphate kinase.
Embodiment 2
[0042] Example 2: Induced expression and purification of polyphosphate kinase
[0043] For the Escherichia coli ajPPK2 constructed in Example 1, pick a single colony on its LB culture plate, inoculate it into a test tube containing 4 ml of 50 μg / mL kanamycin sulfate LB medium, and cultivate it at 37°C for 15 hours to become the first grade For seed solution, inoculate the primary seed solution into a Erlenmeyer flask containing 100ml of TB medium according to the inoculation amount of 1v / v%. When the OD600 is 0.6-0.8, add 0.1mM IPTG and cultivate overnight at 28°C and 200rpm. Then at 4°C, 10000rpm, centrifuge for 10min, collect the bacterial cells respectively, and freeze overnight at -20°C.
[0044] The collected cells were resuspended with 10ml of 0.1M Tris-HCl buffer (pH8.0), and ultrasonically disrupted (200W, super 1.5s, stop 3.5s, 10min in total). Centrifuge at 10,000 rpm for 30 minutes at 4°C to obtain crude enzyme solution of PPK2.
[0045] The crude enzyme liquid wa...
Embodiment 3
[0047] Embodiment 3: the investigation of ATP regeneration catalyzed by crude enzyme
[0048] In order to investigate the practicability of using low-cost crude enzyme solution to catalyze ATP regeneration, the crude enzyme PKK2 in Example 2 was investigated.
[0049] Enzyme reaction system and reaction conditions: 0.1M Tris-HCl buffer (PH8.0), 20mM MgCl2, 2.5mM AMP, 2.5mM sodium hexametaphosphate, after the dissolution is complete, use sodium hydroxide to correct pH8.0, constant volume to 8ml. Then add 2ml of the PKK2 crude enzyme in Example 2, react at 30°C for 2h, and quantitatively analyze AMP, ADP and ATP by HPLC, wherein AMP1mM, ADP1M, ATP0.5mM, that is, the ATP generation rate is 20% .
[0050] The detection results prove that polyphosphate kinase can use sodium hexametaphosphate as a substrate to catalyze the transfer of high-energy phosphate bonds to AMP to generate ADP and further generate ATP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com