Synthesis method of Tafamidis and derivative thereof
A synthesis method and technology of derivatives, applied in the direction of organic chemistry, etc., can solve the problems of many reaction steps, increased cost, low total yield, etc., and achieve the effects of fewer reaction steps, high total yield, and reduced pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
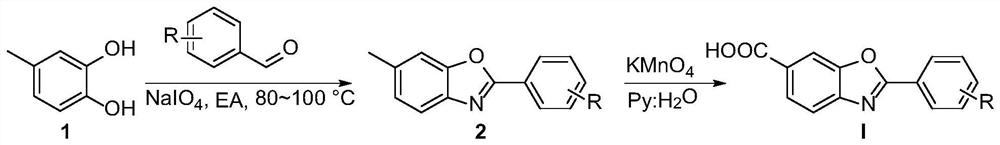
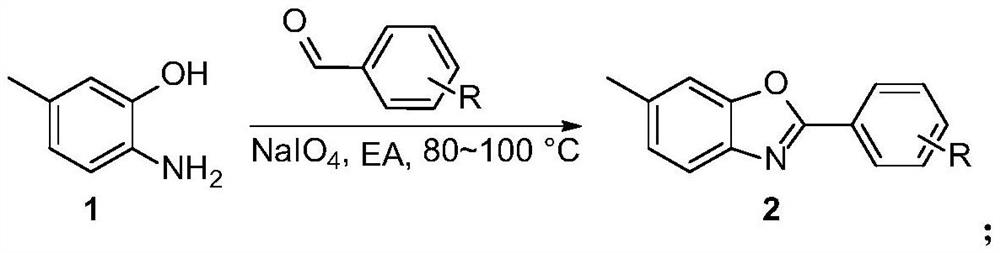
[0034] A kind of synthetic method of Tafamidis and derivative thereof, the preparation raw material of described Tafamidis derivative comprises: 6-amino m-cresol, aldehyde compound, sodium periodate (NaIO 4 ),potassium permanganate.
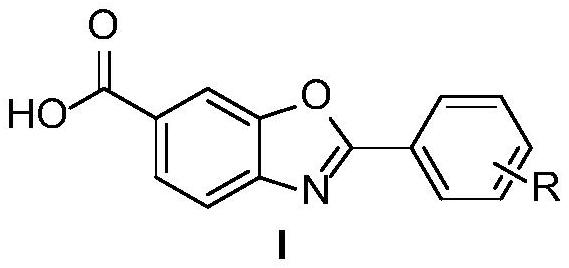
[0035] In one embodiment, the Tafamidis derivative has the following structural formula (I):
[0036]
[0037] In one embodiment, in the structural formula, R is a substituent at any one or more positions on the benzene ring, and each R is independently selected from H, tert-butyl, methoxy, F, Cl, Br, I, trifluoromethyl, nitro, methyl formate.
[0038] In one embodiment, the aldehyde compound is selected from benzaldehyde, p-methoxybenzaldehyde, p-tert-butylbenzaldehyde, p-fluorobenzaldehyde, p-chlorobenzaldehyde, p-bromobenzaldehyde, p-iodine One of benzaldehyde, 3,5-dichlorobenzaldehyde, p-trifluoromethylbenzaldehyde, p-nitrobenzaldehyde, and methyl p-formylbenzoate.
[0039] In one embodiment, preferably, a kind of synthetic method of Ta...
Synthetic example 1
[0061] Synthesis of Tafamidis
[0062] (1) Preparation of 2-(3,5-dichlorophenyl)-6-methylbenzo[d]oxazole: add 1mmol 6-amino-m-cresol, 2mmol 3,5-dichlorobenzaldehyde in the reactor , 2 mmol NaIO 4 , 10 mL EA. Under nitrogen atmosphere, keep stirring at 100°C for 5h, stop the reaction, cool to room temperature, wash with saturated NaCl, then extract with ethyl acetate, distill and concentrate under reduced pressure to remove the solvent, dry, and the crude product is separated by column chromatography to obtain Target product, yield 91%.
[0063] (2) Synthesis of Tafamidis: Then 0.4mmol 2-(3,5-dichlorophenyl)-6-methylbenzo[d]oxazole and 6eq KMnO 4 Added to a mixed solution of pyridine (0.9 mL) and water (0.6 mL). The resulting solution was stirred at room temperature for 30 minutes and heated to boiling (about 100°C). After the reaction was completed, the pH was adjusted to pH=2-3 using 1M HCl solution, and then the product was extracted twice with ethyl acetate and water. ...
Synthetic example 2
[0065] Synthesis of 2-(4-methoxyphenyl)benzo[d]oxazole-6-carboxylic acid
[0066] (1) Preparation of 6-methyl-2-(4-methoxyphenyl)benzo[d]oxazole: add 1mmol6-amino-m-cresol, 2mmol 4-methoxybenzaldehyde, 2mmol NaIO 4 , 10 mL EA. Under nitrogen atmosphere, keep stirring at 100°C for 5h, stop the reaction, cool to room temperature, wash with saturated NaCl, then extract with ethyl acetate, distill and concentrate under reduced pressure to remove the solvent, dry, and the crude product is separated by column chromatography to obtain Target product, yield 87%.
[0067] (2) Synthesis of 2-(4-methoxyphenyl)benzo[d]oxazole-6-carboxylic acid: Then 0.4mmol 6-methyl-2-(4-methoxyphenyl)benzo [d] Oxazole and 6eq KMnO 4 Added to a mixed solution of pyridine (0.9 mL) and water (0.6 mL). The resulting solution was stirred at room temperature for 30 minutes and heated to boiling (about 100°C). After the reaction was completed, the pH was adjusted to pH=2-3 using 1M HCl solution, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com