Human sepsis pathogen detection kit and detection method
A detection kit and technology for sepsis, applied in biochemical equipment and methods, measurement/testing of microorganisms, resistance to vector-borne diseases, etc., can solve the problems of time-consuming, interference of blood culture positive rate and lack of diagnosis in sepsis Sensitivity and specificity and other issues, to achieve good sensitivity and specificity, high clinical application prospects, time and cost advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
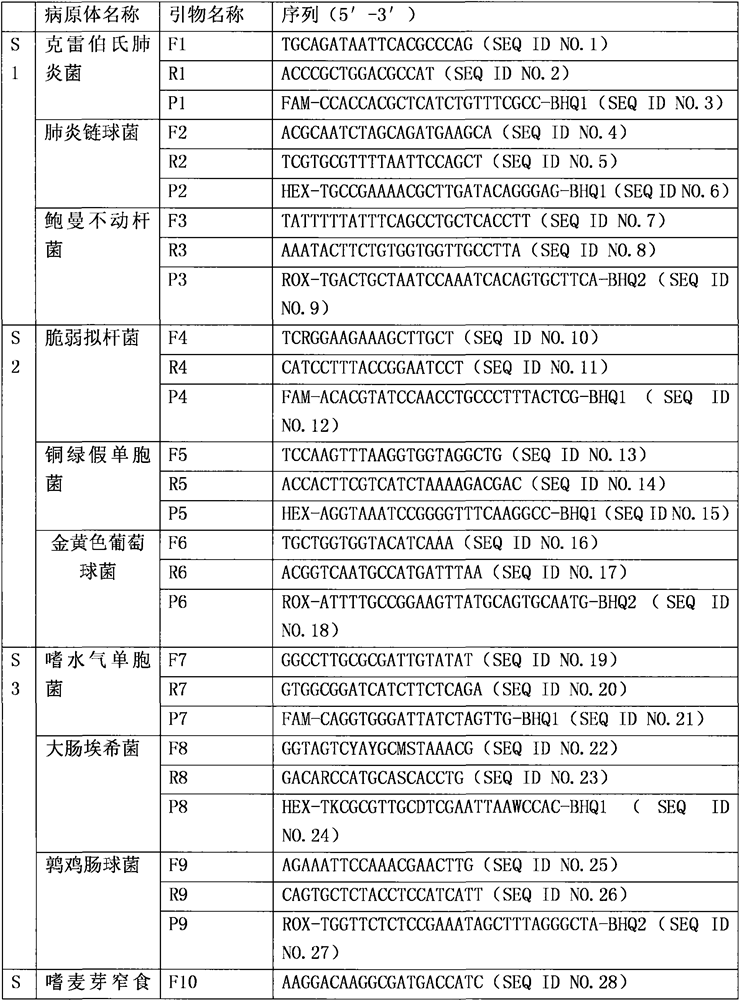
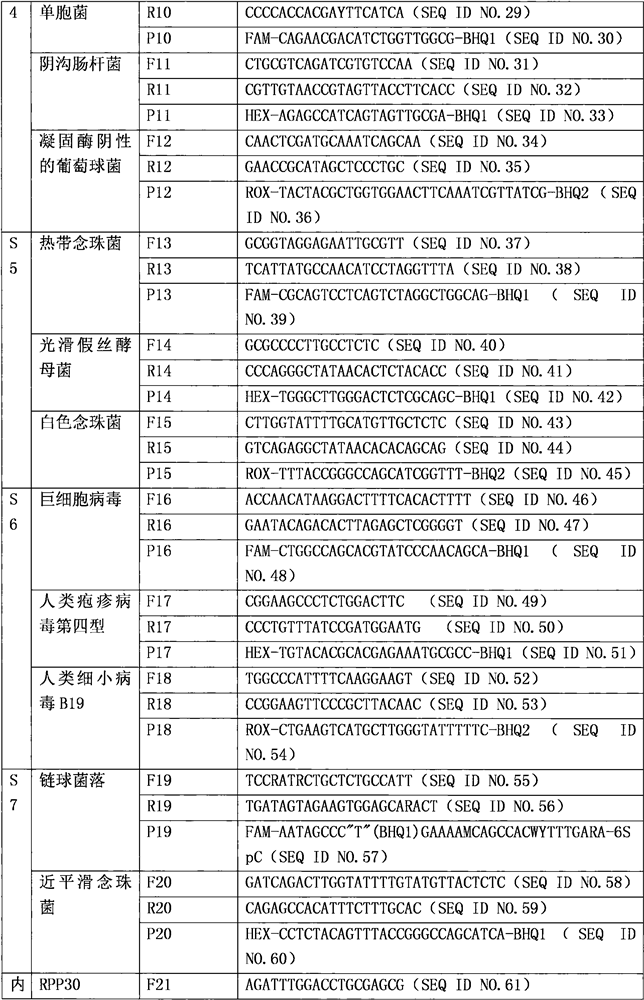
[0022] Embodiment one: the composition of kit
[0023] 1. Specific primer probe
[0024]
[0025]
[0026]
[0027] Note: where W is G or T: R is G or A; Y is C or T; M is A or C; S is G or C; D is A or G or T; K is G or T.
[0028] 2. The PCR reaction reagent is: TaqPath TM 1-Step Multiplex Master Mix (No ROX).
[0029] 3. Deionized water.
Embodiment 2
[0030] Embodiment two: DNA / RNA preparation
[0031] 1ml of human peripheral venous blood was collected according to medical routine, and EDTA was added for anticoagulation. Using Thermo's MagMAX TM CORE Nucleic Acid Purification Kit+MagMAX TM CORE Mastitis&PanbacteriaModule Extraction Kit, extract DNA and RNA.
Embodiment 3
[0032] Embodiment three: PCR reaction system preparation
[0033] Prepare the PCR reaction system according to the following system:
[0034] A total of 7 reaction systems (the total reaction system is 20 μL), which are respectively S1-S7 in the above table, are composed of mixed internal reference primers and probes. PCR reaction reagent: 10 μL; the concentration of each primer and probe is 10M, the volume of primers is 0.8 μL, and the volume of probe is 0.4 μL; sample template: 2 μL; deionized water is enough to make up 20 μL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com