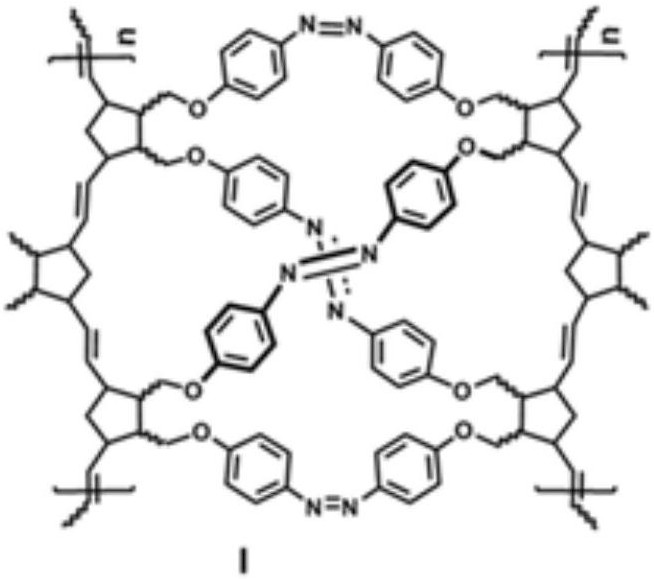
Polynorbornene porous material containing azobenzene structure and preparation method thereof
A technology of polynorbornene and porous materials, applied in the field of materials, can solve the problems of poor functional group tolerance, small molecular weight, long time for potassium hydroxide-mediated coupling polymerization reaction, etc., and achieves the effect of high efficiency and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
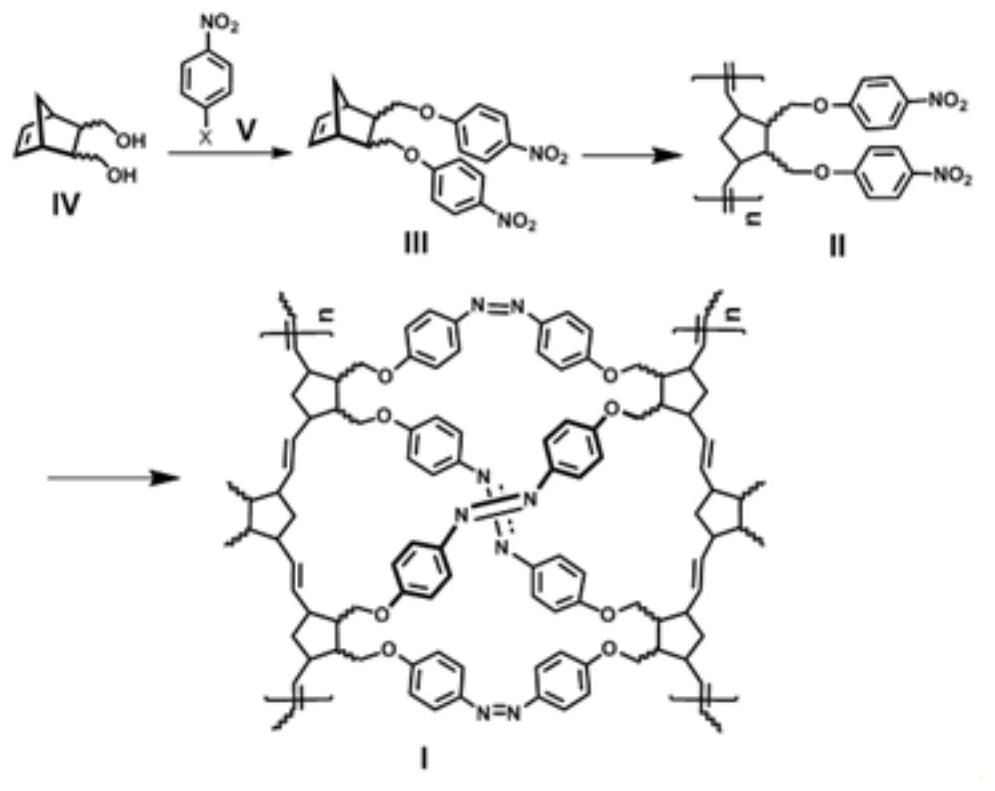
[0060] Such as figure 2 As shown, a flow chart of a preparation method of a polynorbornene porous material containing an azobenzene structure provided by the present invention may specifically include:
[0061] Step 1, preparing disubstituted norbornene p-nitrophenyl ether III: using norbornene diol shown in structural formula IV and nitrohalobenzene shown in structural formula V to prepare disubstituted norbornene p-nitrophenyl ether shown in structural formula III Nitrophenyl ether;
[0062] Step 2, preparation of polynorbornene p-nitrophenyl ether II: the disubstituted norbornene p-nitrophenyl ether shown in the structural formula III is prepared by olefin ring-opening metathesis polymerization under the action of a ruthenium metal catalyst Polynorbornene p-nitrophenyl ether shown in II;
[0063] Step 3, preparing polynorbornene porous material containing azobenzene structure I: the polynorbornene p-nitrophenyl ether shown in the structural formula II and a reducing agen...
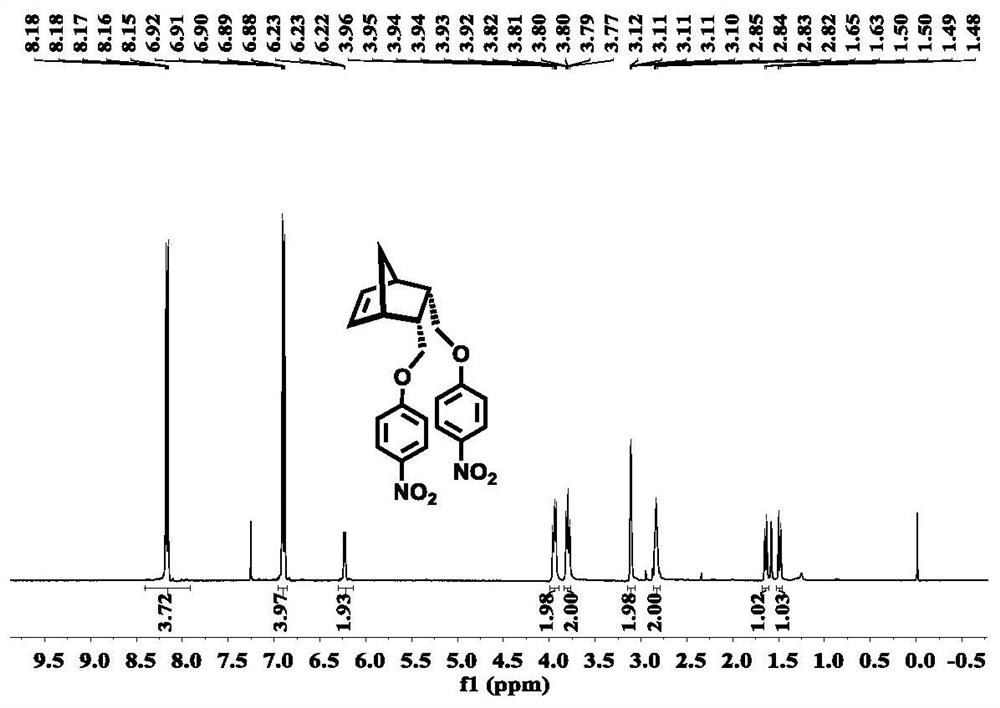
Embodiment 1
[0067] Example 1, Preparation of azobenzene-linked polynorbornene porous material in Endo-configuration
[0068] Step 1: Prepare Norbornenediol of Endo-configuration with reference to related technology
[0069] Measure DCPD (30mL, 0.35mol) in a 100mL flask with a measuring cylinder, heat up to 200°C, distill and condense to obtain cyclopentadiene; Cis-1,4-butenediol (18mL, 0.2mol) was placed in a 250mL sealed reaction tube, heated to 210°C for 6h. Cool to room temperature to precipitate white crystals, obtain endo-configuration norbornenediol (14g, 45%) by filtration, dissolve the filtrate in hot water (100ml, about 80°C), separate the liquid with a separatory funnel, and dissolve the water The phase was concentrated under reduced pressure, and the oil was recrystallized to obtain endo-configured norbornene diol (7.0g, 23%). The two recrystallizations produced a total of endo-configuration norbornene diol (21g, Overall yield 68%).
[0070] Wherein, the DCPD is a cyclopenta...
Embodiment 2
[0089] Example 2, Synthesis of azobenzene-linked polynorbornene porous material in Endo-configuration
[0090] Step 1: Preparation of Norbornenediol in Endo-configuration
[0091] Weigh norbornene anhydride (VI, 4.9g, 30mmol, 1equiv.) in endo configuration, add it into anhydrous tetrahydrofuran (100mL) and stir to dissolve, place it in an ice-water bath to cool; weigh LiAlH 4 (3.6g, 90mmol, 3equiv.) and slowly added to the above mixture, maintained at low temperature and stirred for 0.5 hours, then slowly raised to room temperature and stirred for 12 hours. The reaction was cooled in an ice-water bath, ice water (8 mL) was slowly added to quench the reaction, and then 10% sodium hydroxide solution (10 mL) was slowly added. After the reaction was quenched, suction filtration under reduced pressure was carried out, the filter residue was washed with ethyl acetate (3x 50mL), and the filtrate was washed with anhydrous MgSO 4 Dried, concentrated and dried with a rotary evaporator...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Total pore volume | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com