Aryl-containing polymer oxidative degradation method and application thereof
An oxidative degradation and polymer technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of slow natural degradation rate, low solubility, serious problems, etc., and achieve wide applicability of substrates and simple operation , the effect of mild oxidation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Degrade polystyrene, a high molecular polymer containing aromatic ring, and selectively oxidize it into acid
[0059]
[0060] In a dry reaction tube, add aromatic ring-containing high molecular polymer 1a (0.5mmol, calculated as monomer, purchased from Aladdin, product number P107085, Mw=146kg / mol), iron compound Fe(OTf) 3 (0.02mmol), additive tetrabutylammonium chloride (0.04mmol), additive hydrochloric acid (0.5mmol) and dichloromethane (2mL), stir and dissolve, mix well, fill with oxygen, place the reaction tube in the light of 400nm ( hv) under irradiation for 48 h with constant stirring. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a crude product, which was separated and purified by dichloromethane / petroleum ether silica gel column chromatography to obtain compound 2a, and the conversion rate of the polymer was measured to be 100%. Yield 56%.
[0061] In this embodiment, the catalyst is ferric ...
Embodiment 2
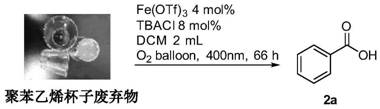
[0062] Example 2: Degradation of waste foam containing aromatic rings, selective oxidation into acids
[0063] In a dry reaction tube, add waste foam 1b (0.5mmol, calculated as monomer, Mw=222kg / mol), iron compound Fe(OTf) 3 (0.02mmol), additives tetrabutylammonium chloride (0.04mmol) and dichloromethane (2mL), stirring and dissolving, mixing evenly, filling with oxygen, placing the reaction tube under 400nm light (hv) for 66h and continuously Stirring, until the reaction was completed, the solvent was distilled off under reduced pressure to obtain a crude product, which was separated and purified by dichloromethane / petroleum ether silica gel column chromatography to obtain compound 2a. The polymer conversion rate was 100%, and the yield was 49% ( figure 1 ).
[0064] In this embodiment, the catalyst is ferric iron. Under the reaction conditions, the forms of iron such as zero-valent iron and divalent iron are easily oxidized to ferric iron. Therefore, different forms of zero...
Embodiment 3
[0065] Example 3: Degrading waste plastic cups containing aromatic rings and selectively oxidizing them into acids
[0066] In a dry reaction tube, add waste plastic cup 1c (0.5mmol, calculated as monomer, Mw=166kg / mol), iron compound Fe(OTf) 3 (0.02mmol), additives tetrabutylammonium chloride (0.04mmol) and dichloromethane (2mL), stirring and dissolving, mixing evenly, filling with oxygen, placing the reaction tube under 400nm light (hv) for 66h and continuously Stirring, until the reaction was completed, the solvent was distilled off under reduced pressure to obtain a crude product, which was separated and purified by dichloromethane / petroleum ether silica gel column chromatography to obtain compound 2a, and the polymer conversion rate was 100%, and the yield was 51% ( figure 2 ).
[0067] In this embodiment, the catalyst is ferric iron. Under the reaction conditions, the forms of iron such as zero-valent iron and divalent iron are easily oxidized to ferric iron. Therefore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com