Polyamide-imide containing alicyclic group and preparation method of polyamide-imide
A polyamide and imide technology, applied in the field of functional polymer materials, can solve the problems of limiting the application of polyimide, high thermal expansion of polyimide, and high molecular chain strength, and achieve good light transmittance and solubility. , good mechanical properties and heat resistance, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
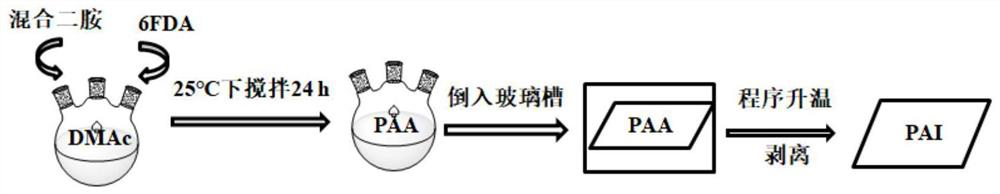
[0039] Further, a preparation method of polyamide-imide containing alicyclic group, the specific operation steps are as follows:
[0040] (2.1), the synthesized NCHABA and TFDB are added into a three-necked flask according to a certain molar ratio, then a solvent is added, nitrogen gas is passed, and magnetic stirring is carried out under an ice bath to obtain a mixed diamine solution;
[0041] (2.2), adding the dianhydride monomer: 6FDA into the obtained mixed diamine solution three times, after the dianhydride monomer is completely dissolved, continue to react for 24 hours at room temperature, thereby obtaining a polyamic acid solution;
[0042] (2.3), then pour the obtained polyamic acid solution into the glass tank, place it in a vacuum oven and carry out temperature programming, thereby obtaining polyamide-imide;
[0043] (2.4), finally stripping the obtained polyamide-imide from the glass tank to finally obtain film-like polyamide-imide containing alicyclic groups.
[0...
Embodiment 1
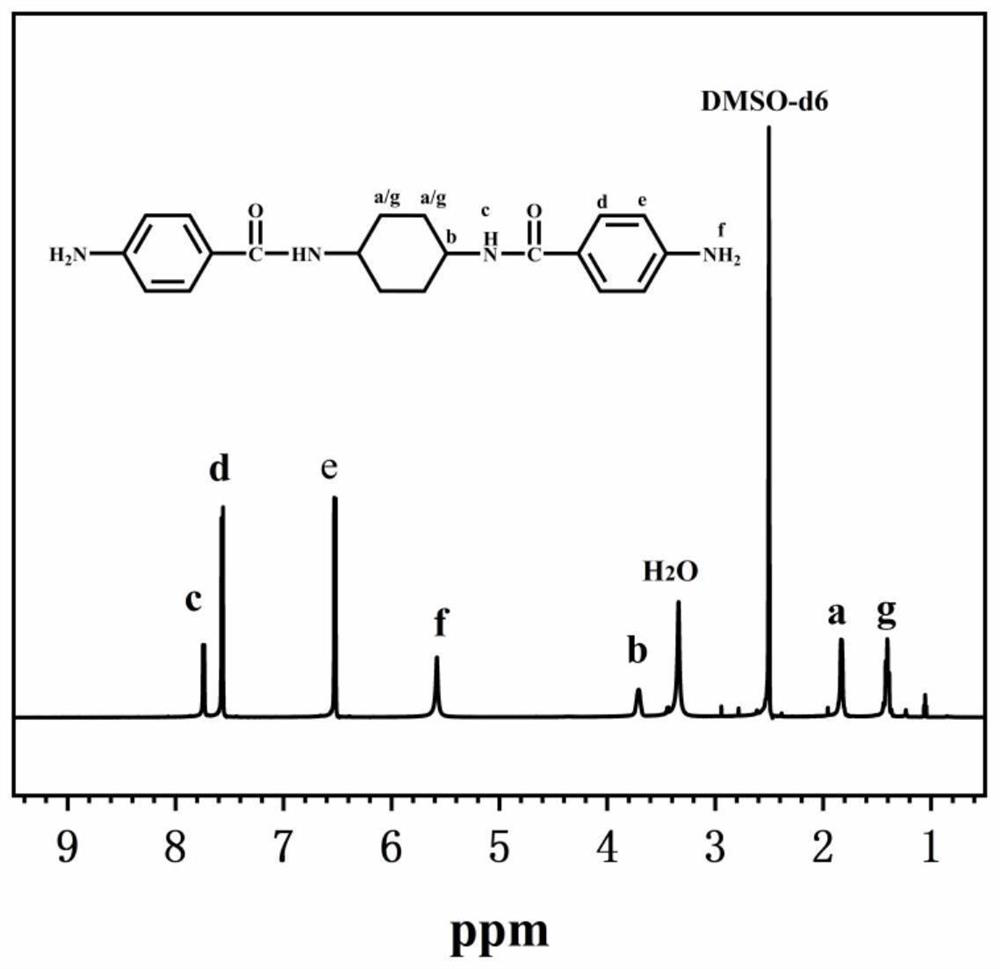
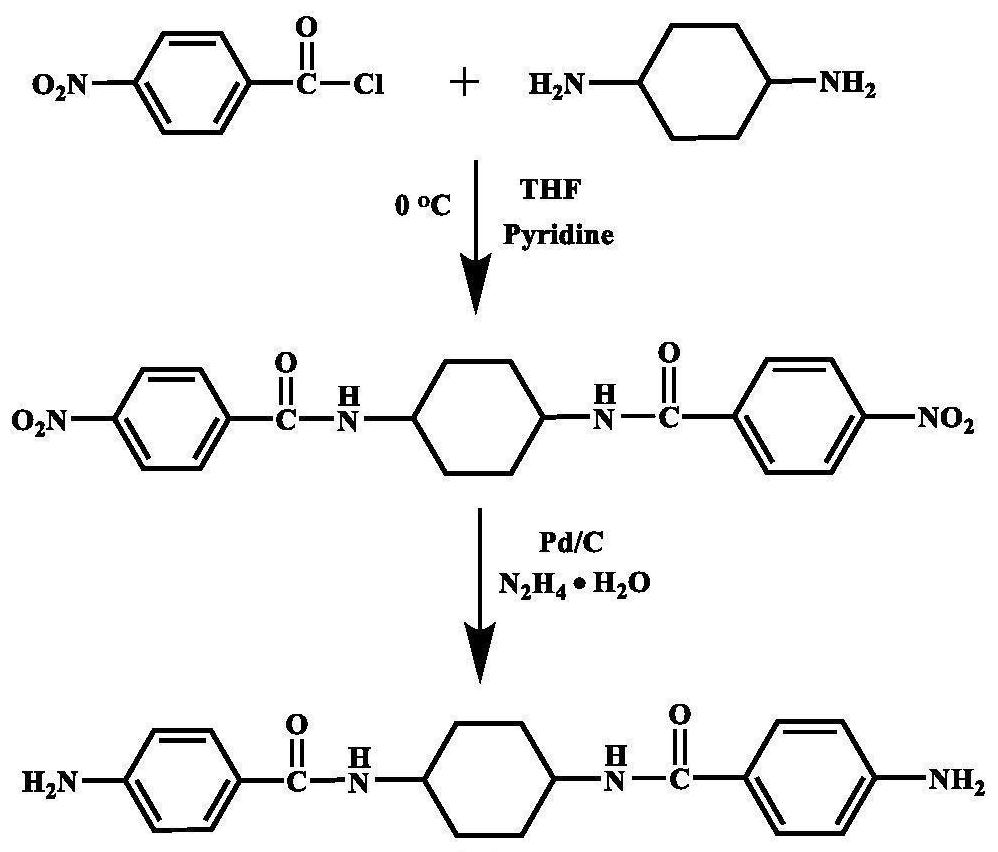
[0057] In a 500ml three-necked flask equipped with a nitrogen inlet and magnetic stirring, add 29.69g (0.16mol) p-nitrobenzoyl chloride, 42.66g (0.32mol) anhydrous aluminum trichloride and 350ml N,N-dimethylethyl Amide (DMAc) solution, ice bath, after magnetic stirring for half an hour, add 9.13g (0.08mol) trans-1,4-cyclohexanediamine solution dissolved in 50ml DMAc with a syringe, react in ice bath for 5h, then continue to react at room temperature 24h; after the reaction is over, pour the reaction solution into 500ml deionized water for precipitation, filter to obtain a filter cake, wash with deionized water and ethanol, and dry in vacuum for 12h to obtain N,N'-(cyclohexane-1,4- Diyl) bis(4-nitrobenzamide); Weigh 4.12g (0.01mol) of the dinitro compound synthesized above, 35ml DMAc and place it in a 250ml three-neck flask equipped with a condenser tube and nitrogen inlet, add 0.124g (3wt%) Pd / C is used as catalyzer; Under 120 ℃ of magnetic agitation, slowly drip 15ml hydrazin...
Embodiment 2
[0060] In a 500ml three-necked flask equipped with a nitrogen inlet and magnetic stirring, add 29.69g (0.16mol) p-nitrobenzoyl chloride, 42.66g (0.32mol) anhydrous aluminum trichloride and 350ml N,N-dimethylethyl Amide (DMAc) solution, ice bath, after magnetic stirring for half an hour, add 9.13g (0.08mol) trans-1,4-cyclohexanediamine solution dissolved in 50ml DMAc with a syringe, react in ice bath for 5h, then continue to react at room temperature 24h; after the reaction is over, pour the reaction solution into 500ml deionized water for precipitation, filter to obtain a filter cake, wash with deionized water and ethanol, and dry in vacuum for 12h to obtain N,N'-(cyclohexane-1,4- Diyl) bis(4-nitrobenzamide); Weigh 4.12g (0.01mol) of the dinitro compound synthesized above, 35ml DMAc and place it in a 250ml three-neck flask equipped with a condenser tube and nitrogen inlet, add 0.124g (3wt%) Pd / C is used as catalyzer; Under 120 ℃ of magnetic agitation, slowly drip 15ml hydrazin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com