Fluorine-containing aromatic diamine compound, preparation method thereof and colorless transparent polyimide film
A technology for transparent polyimide and aromatic diamine, which is used in the preparation of amino compounds from amines, organic chemistry, etc., can solve problems such as unfavorable promotion and application, increasing the difficulty of compound purification, production cost, and restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The present invention provides a method for preparing a fluorine-containing aromatic diamine compound described in the above technical scheme, comprising the following steps:
[0060] Carrying out a coupling reaction between an amino-substituted phenylboronic acid (ester) having a structure of formula I and a fluorine-containing halogenated compound having a structure of formula II to obtain a fluorine-containing aromatic diamine compound having a structure of formula V;
[0061] Or carry out coupling reaction between the benzene diboronic acid (ester) with the structure of formula III and the fluorine-containing halogenated amino compound with the structure of formula IV to obtain the fluorine-containing aromatic diamine compound with the structure of formula V'.
[0062] The method provided by the invention efficiently synthesizes fluorine-containing aromatic diamine compounds in one step, and has the obvious advantages of simple route, simple operation, high reaction ...
Embodiment 1
[0124] 1. Synthesis of fluorine-containing diamine monomer
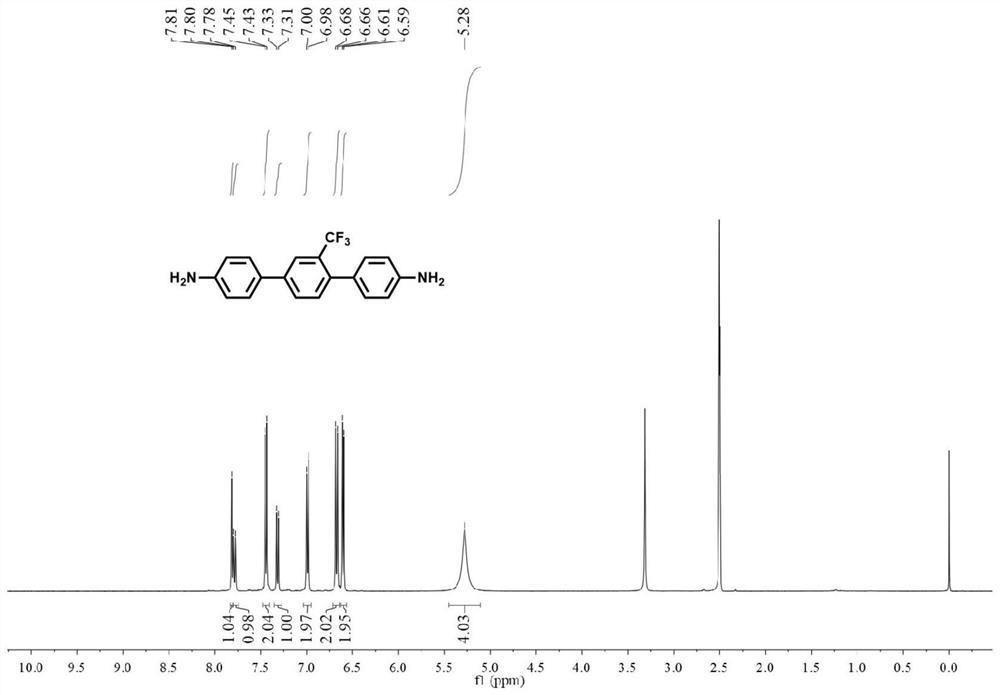
[0125] Under argon protection, 4-aminophenylboronic acid pinacol ester (0.022mol, 4.82g), 2,5-dibromobenzotrifluoride (0.01mol, 3.04g), tetrakis (triphenylphosphine) palladium ( 0.001mol, 1.16g), anhydrous potassium carbonate (0.1mol, 13.82g), and 60mL toluene were added to a 250mL Schlenk bottle in turn, and the reaction system was stirred and refluxed at 120°C for at least 15h, then the heating was stopped, and the solvent was extracted and evaporated After purification, 2.92 g of off-white solid was finally obtained, namely 1,4-bis(4-aminophenyl)-2-trifluoromethylbenzene, with a yield of 89%. 1 H NMR (400MHz, DMSO-d 6 )δ7.81(s,1H),7.79(d,J=8.0Hz,1H),7.44(d,J=8.4Hz,2H),7.32(d,J=8.0Hz,1H),6.99(d, J=8.4Hz, 2H), 6.67(d, J=8.4Hz, 2H), 6.60(d, J=8.4Hz, 2H), 5.28(s, 4H).
[0126] The molecular structural formula of fluorine-containing diamine in the present embodiment is as follows:
[0127]
[0128] 2. Preparatio...
Embodiment 2
[0133] 1. Synthesis of fluorine-containing diamine monomer
[0134] Under nitrogen protection, 1,4-benzenediboronic acid (0.01mol, 1.66g), 4-bromo-3-(trifluoromethyl)aniline (0.02mol, 2.40g), tetrakis (triphenylphosphine) palladium (0.0008mol, 0.93g), anhydrous potassium carbonate (0.1mol, 13.82g), and 60mL dioxane were added to a 250mL Schlenk bottle in turn, and the reaction system was stirred and refluxed at 130°C for at least 12h, then the heating was stopped, and after extraction , Evaporate the solvent and purify to finally obtain 3.45 g of pure white solid, that is, 1,4-bis(2-trifluoromethyl-4-aminophenyl)benzene, with a yield of 87%. 1 H NMR (400MHz, DMSO-d 6 )δ7.23(s,4H),7.06(d,J=8.4Hz,2H),6.98(d,J=2.0Hz,2H),6.83(dd,J=8.4,2.0Hz,2H),5.63( s,4H).
[0135] The molecular structural formula of fluorine-containing diamine in the present embodiment is as follows:
[0136]
[0137] 2. Preparation of polyimide film
[0138] Under nitrogen protection, 1,4-bis(2-trifluor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com