Preparation and application of positive electrode material of chargeable and dischargeable lithium ion battery
A technology of lithium ion battery and positive electrode material, which is applied in the field of preparation and application of positive electrode material of rechargeable and dischargeable lithium ion battery, and can solve the problems of limiting the application of fluorinated carbon materials and not having rechargeable and discharging properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
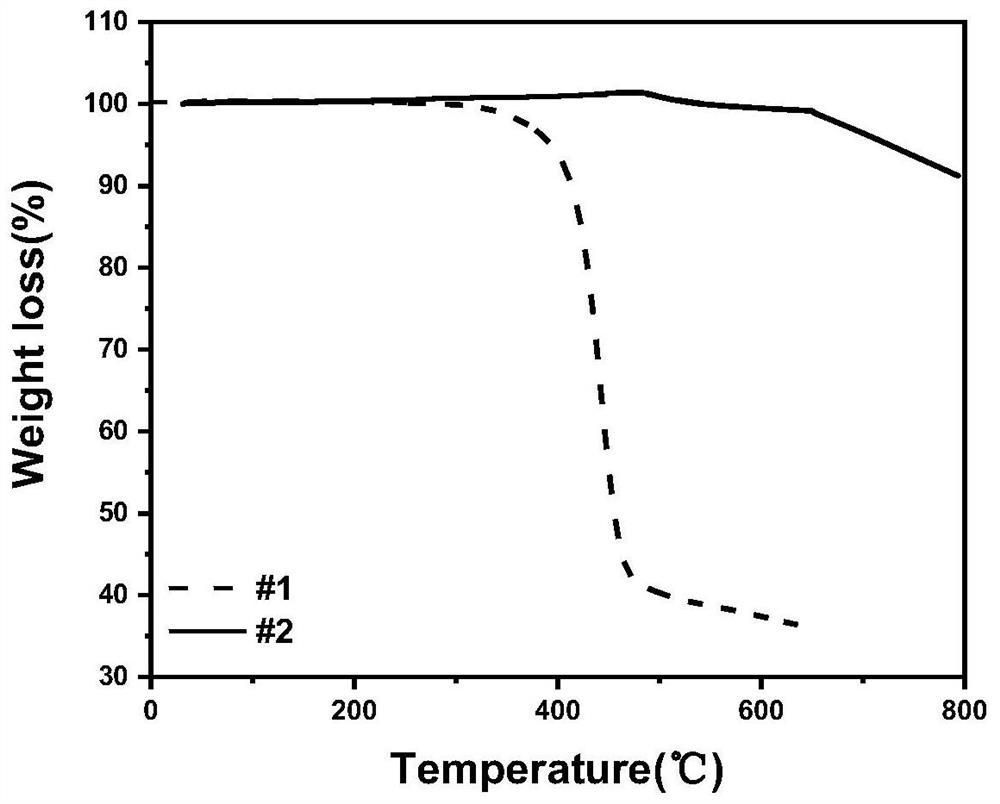
Image
Examples
Embodiment 1
[0041] Add 3 parts by weight of polyacrylonitrile powder to 50 parts by weight of dimethylformamide and stir at room temperature (25°C, the same below) to fully obtain a clear dispersion, then add 7 parts by weight of graphite fluoride powder Add 1 weight part of lithium carbonate to the above dispersion liquid and stir at 90°C with a stirring speed of 500r / min to fully obtain a slurry, and spray the obtained slurry to obtain uniformly dispersed particles. The inlet temperature of the spray chamber is 190°C , and then calcined the obtained particles in an argon atmosphere at 600° C. for 4 hours to obtain a carbon fluoride / polyacrylonitrile composite material.
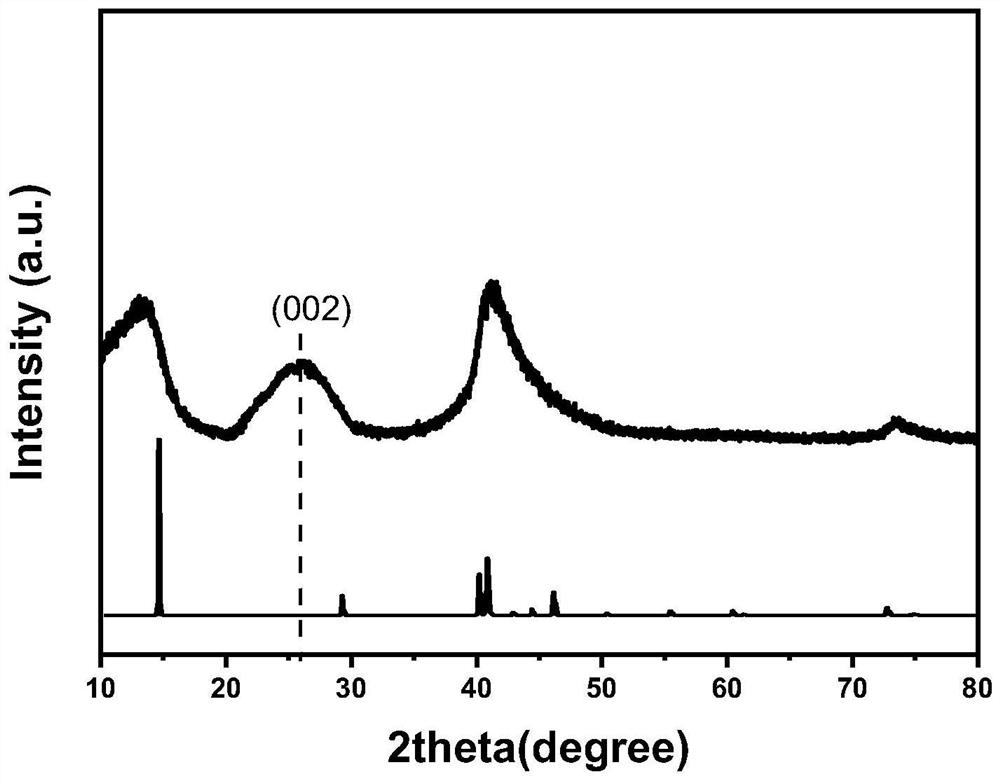
[0042] The XRD figure of the carbon fluoride / polyacrylonitrile composite material prepared in this embodiment is as follows figure 1 shown, from figure 1 It can be seen that no diffraction peak of polyacrylonitrile is found at 16.8° in the XRD diagram, indicating that polyacrylonitrile has become an amorphous phase whe...
Embodiment 2
[0059] The mass ratio of graphite fluoride powder and polyacrylonitrile powder in Example 1 is adjusted to 5:5, and other preparation conditions remain unchanged; the prepared carbon fluoride / polyacrylonitrile composite material is prepared into a positive electrode sheet and assembled into a battery, and test the charge and discharge performance of the obtained battery with a battery test system, the preparation, assembly and test methods are the same as in Example 1.
[0060] The charge-discharge performance test result of the assembled battery of embodiment 2 is as follows Figure 9 shown, from Figure 9 It can be seen from the results that the battery assembled with the composite material prepared in this example has a discharge specific capacity of only 497.1mAh / g in the first cycle of the charge and discharge test, although the discharge specific capacity of the second cycle reaches 331.6mAh / g, which is higher than that prepared in Example 1. The discharge specific capa...
Embodiment 3
[0066] Polymer is changed into polypyrrole in embodiment 1:
[0067] Add 5 parts by weight of pyrrole monomer and 5 parts by weight of carbon fluoride into 50 parts by weight of deionized water and stir at room temperature to obtain a dispersion, slowly add ferric chloride solution dropwise to the dispersion, and The reaction was stirred for 12 hours, and the stirring temperature was controlled at 2°C; finally, the obtained reaction system was filtered, washed, and dried in a vacuum environment at a drying temperature of 60°C and a drying time of 12 hours to obtain a carbon fluoride / polypyrrole composite material.
[0068] The carbon fluoride / polypyrrole composite material prepared in this example was prepared into a positive electrode sheet and assembled into a battery, and the charge and discharge performance of the obtained battery was tested with a battery test system. The preparation, assembly methods and test methods were the same as in Example 1. The test results of cha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Charge and discharge rate | aaaaa | aaaaa |
| Specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com