Continuous preparation method of alicyclic epoxy compound
A technology of cycloaliphatic epoxy and compounds, applied in chemical instruments and methods, chemical/physical/physical chemical processes, chemical/physical/physical chemical reactors, etc. Problems such as complex process, high reaction conversion rate and yield, low process risk, and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
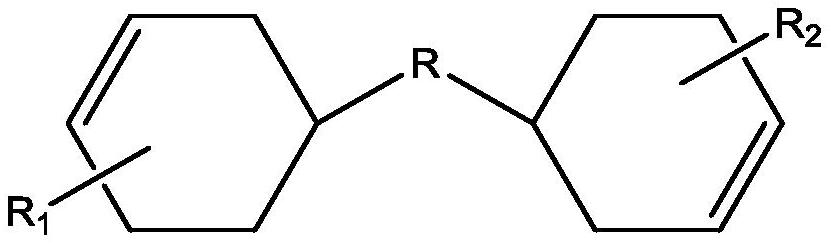
[0023] As described in the background art section, the prior art has the problems of low oxidation activity of oxidizing agents, complicated operation process and low conversion rate of conventional static microtubular reactors. In order to solve this problem, the present invention provides a continuous preparation method of cycloaliphatic epoxy compounds, which includes the following steps: S1, passing alicyclic olefin and hydrogen peroxide aqueous solution continuously into a microemulsifier for emulsification to form an emulsion , wherein the concentration of hydrogen peroxide aqueous solution ≥ 8wt%; S2, the emulsion is continuously passed into the microreactor for oxidation reaction to obtain the crude mother liquor; S3, the crude mother liquor is purified to obtain the alicyclic epoxy compound.
[0024] The present invention adopts a dynamic microtubular reactor, ensures the homogeneity of the reaction system through the combination of a microemulsifier and a microreactor...
Embodiment 1
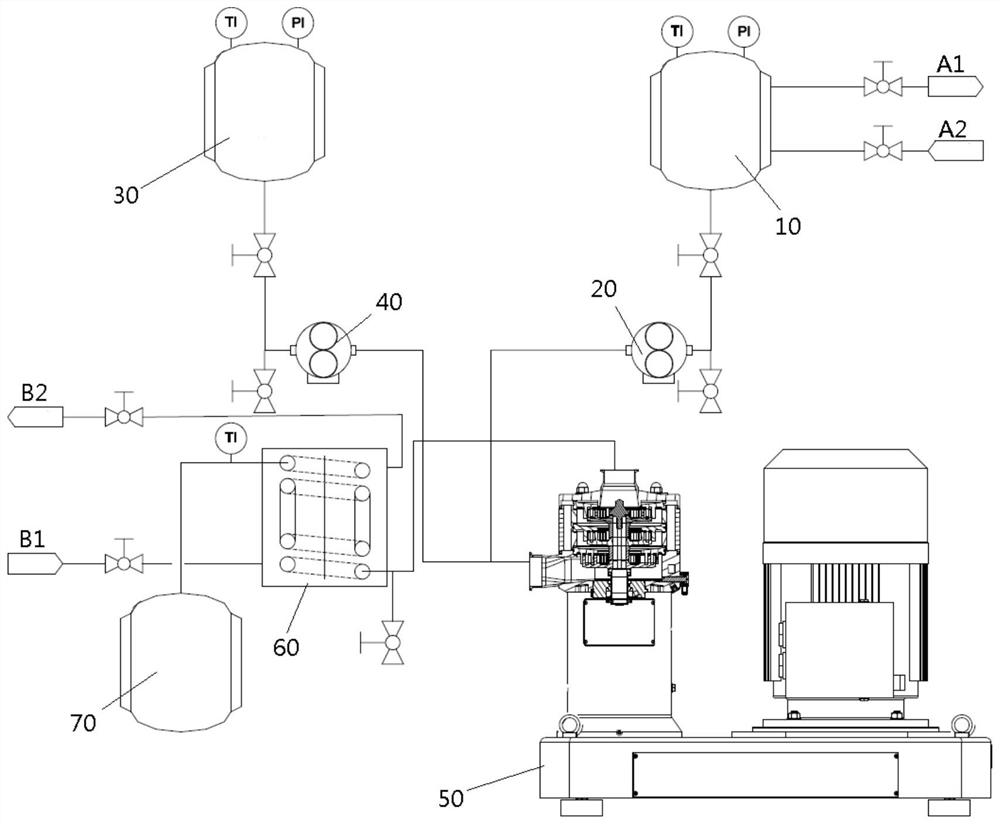
[0047] Such as figure 1 As shown, in the alicyclic olefin feeding tank 10, 4-(2-cyclohex-3-en-1-ylpropyl-2-yl) cyclohexene 2040g, catalyst cetylpyridinium phosphotungstic acid quaternary ammonium salt 100g, 1200g of chloroform, and 150g of sodium dihydrogen phosphate are passed into the microemulsifier 50 with a flow rate of 10L / h by the first metering pump 20 under stirring conditions, and during this period, when it reaches the microemulsifier 50 through pipeline heating The temperature reaches 60-70°C. Simultaneously, the hydrogen peroxide aqueous solution feed tank 30 that 27% hydrogen peroxide aqueous solution 2510g is housed passes in the microemulsifier 50 with the flow rate of 5.8L / h through the second metering pump 40, and during this period, when it reaches the microemulsifier 50 by pipeline heating The temperature reaches 30-40°C. During the continuous feeding process, the double bond in the alicyclic olefin and the H in the hydrogen peroxide aqueous solution 2 o...
Embodiment 2
[0049] The difference with Example 1 is that the flow rate of the hydrogen peroxide aqueous solution is controlled to be 4.3L / h, and in the continuous feeding process, the double bond in the alicyclic olefin and the H in the hydrogen peroxide aqueous solution 2 o 2 The molar ratio is 1:0.7, and the mother liquor flowing out from the upper part enters the microreactor at a flow rate of 14.3L / h. Final product viscosity 90cps / 25°C, epoxy equivalent 256g / mol; olefin conversion 70%, 4-(2-cyclohex-3-en-1-ylpropyl-2-yl)epoxycyclohexane 35% , 4-(2-cyclohex-3-epoxy-1-ylpropyl-2-yl)epoxycyclohexane 33%, total yield 87%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy equivalent | aaaaa | aaaaa |
| Epoxy equivalent | aaaaa | aaaaa |
| Epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com