Method for deeply removing oxygen in hydrogen through electrocatalysis at normal temperature and pressure to obtain high-purity hydrogen
An electrocatalytic, high-purity technology, applied in chemical instruments and methods, separation methods, electrodes, etc., can solve the problems of high cost of precious metal Pt, high reaction temperature, low palladium element content, etc., and achieve the effect of broad practical application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
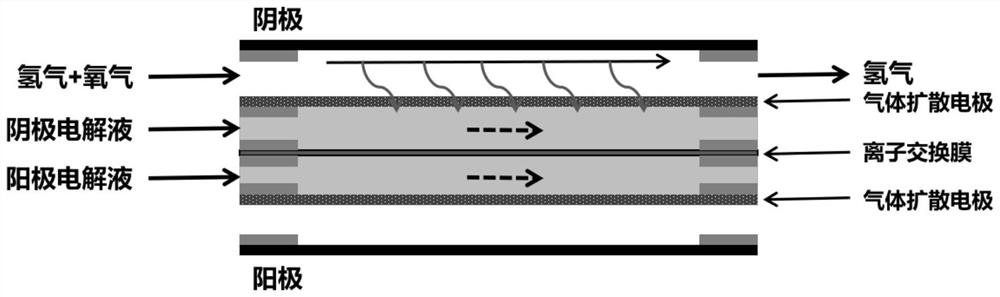
[0030] (1) The gas diffusion electrode made of Pt nanoparticles is used as the cathode of the electrolytic cell; the gas diffusion electrode made of iridium oxide catalyst is used as the anode of the electrolytic cell; both the catholyte and the anolyte are 0.5M sulfuric acid solution, the separated by a proton exchange membrane, and figure 1 The schematic diagram of the apparatus shown assembles the various components.
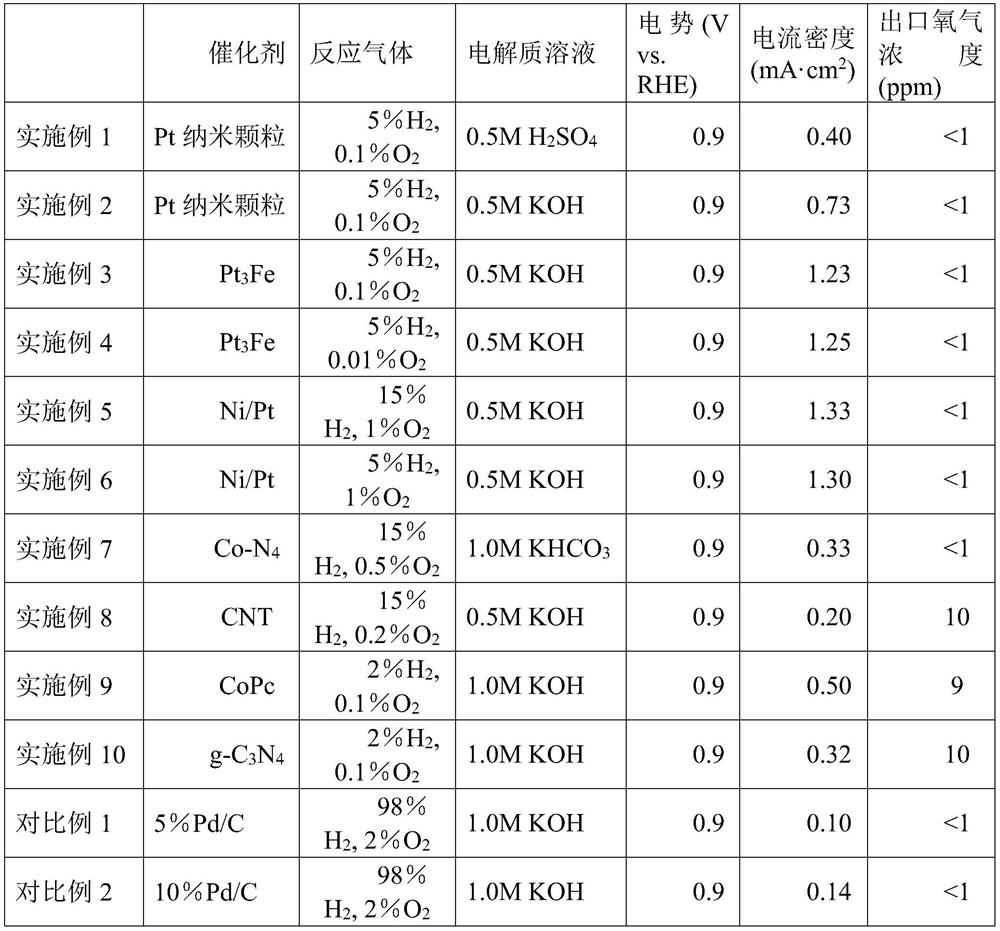
[0031] (2) The flow rate of the oxygen-containing hydrogen mixed reaction gas controlled by a gas mass flowmeter is 50 sccm, and the oxygen-containing hydrogen mixed gas used in the experiment consists of: 5% hydrogen, 0.1% oxygen, and the rest is argon.
[0032] (3) A peristaltic pump is used to control the flow rate of catholyte and anolyte to 50 sccm.
[0033] (4) The catalytic activity of Pt nanoparticles was characterized by potentiostatic method, and the oxygen content in the outlet gas was determined by online gas chromatography.
Embodiment 2
[0035](1) The gas diffusion electrode made of Pt nanoparticles is used as the cathode of the electrolytic cell; the gas diffusion electrode made of iridium oxide catalyst is used as the anode of the electrolytic cell; both the catholyte and the anolyte are 0.5M KOH solution, with anion exchange membrane separation, and as figure 1 The schematic diagram of the apparatus shown assembles the various components.
[0036] (2) The flow rate of the oxygen-containing hydrogen mixed reaction gas controlled by a gas mass flowmeter is 50 sccm, and the oxygen-containing hydrogen mixed gas used in the experiment consists of: 5% hydrogen, 0.1% oxygen, and the rest is argon.
[0037] (3) A peristaltic pump is used to control the flow rate of catholyte and anolyte to 50 sccm.
[0038] (4) The catalytic activity of Pt nanoparticles was characterized by potentiostatic method, and the oxygen content in the outlet gas was determined by online gas chromatography.
Embodiment 3
[0040] (1) Pt 3 The gas diffusion electrode made of Fe is used as the cathode of the electrolytic cell; the gas diffusion electrode made of iridium oxide catalyst is used as the anode of the electrolytic cell; the catholyte and the anolyte are both 0.5M KOH solution, separated by an anion exchange membrane, and Such as figure 1 The schematic diagram of the apparatus shown assembles the various components.
[0041] (2) The flow rate of the oxygen-containing hydrogen mixed reaction gas controlled by a gas mass flowmeter is 50 sccm, and the oxygen-containing hydrogen mixed gas used in the experiment consists of: 5% hydrogen, 0.1% oxygen, and the rest is argon.
[0042] (3) A peristaltic pump is used to control the flow rate of catholyte and anolyte to 50 sccm.
[0043] (4) Using the potentiostatic method Pt 3 The catalytic activity of Fe was determined, and the oxygen content in the outlet gas was determined by online gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com