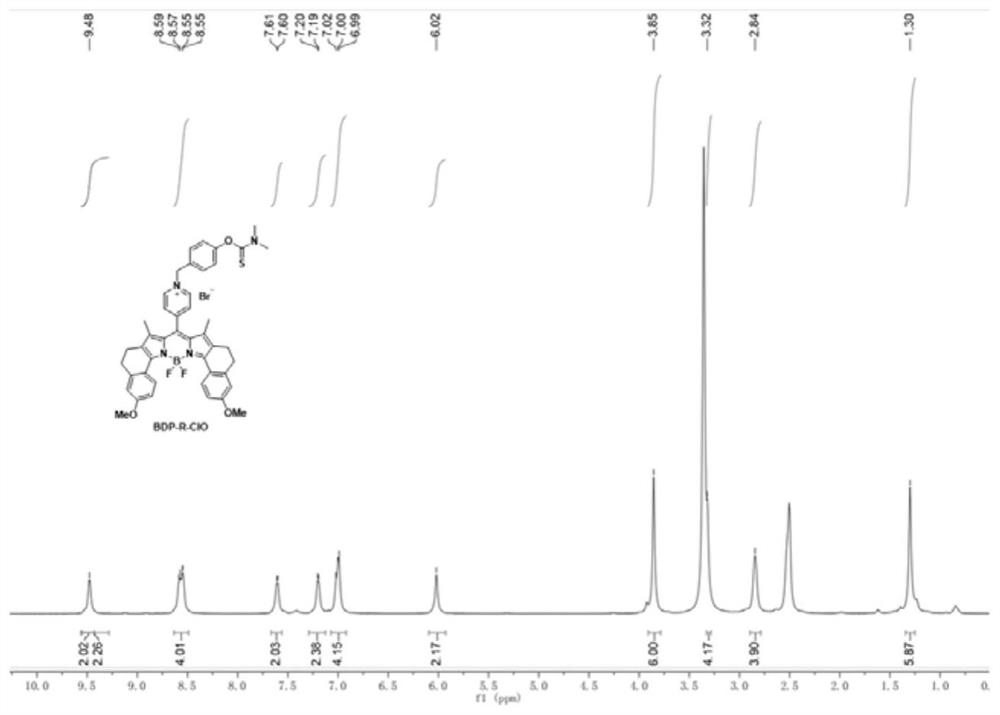
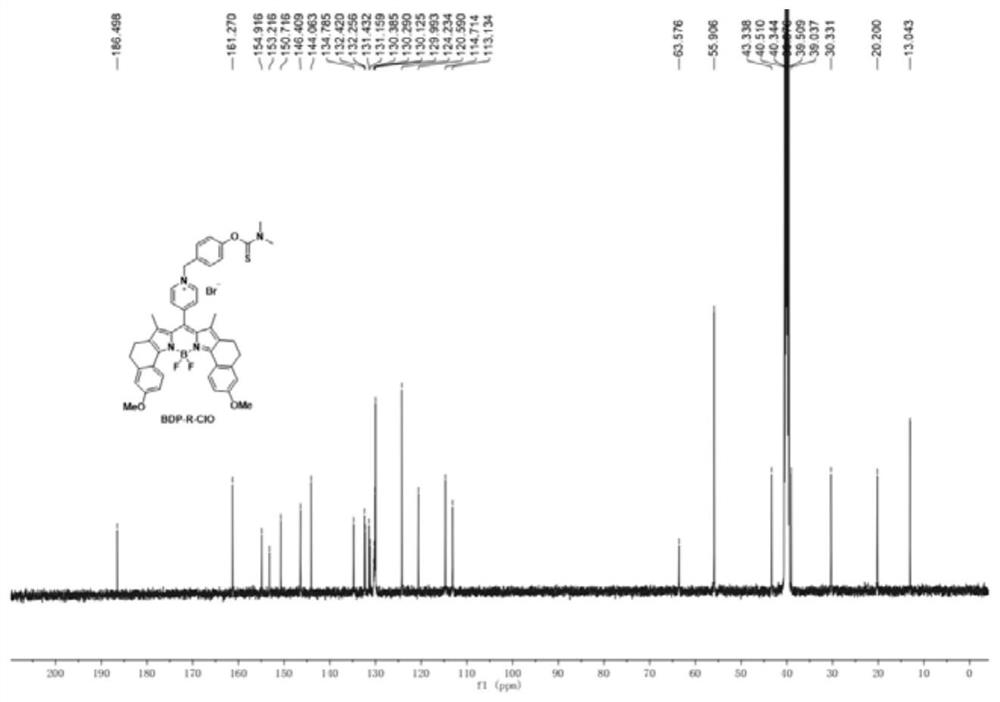
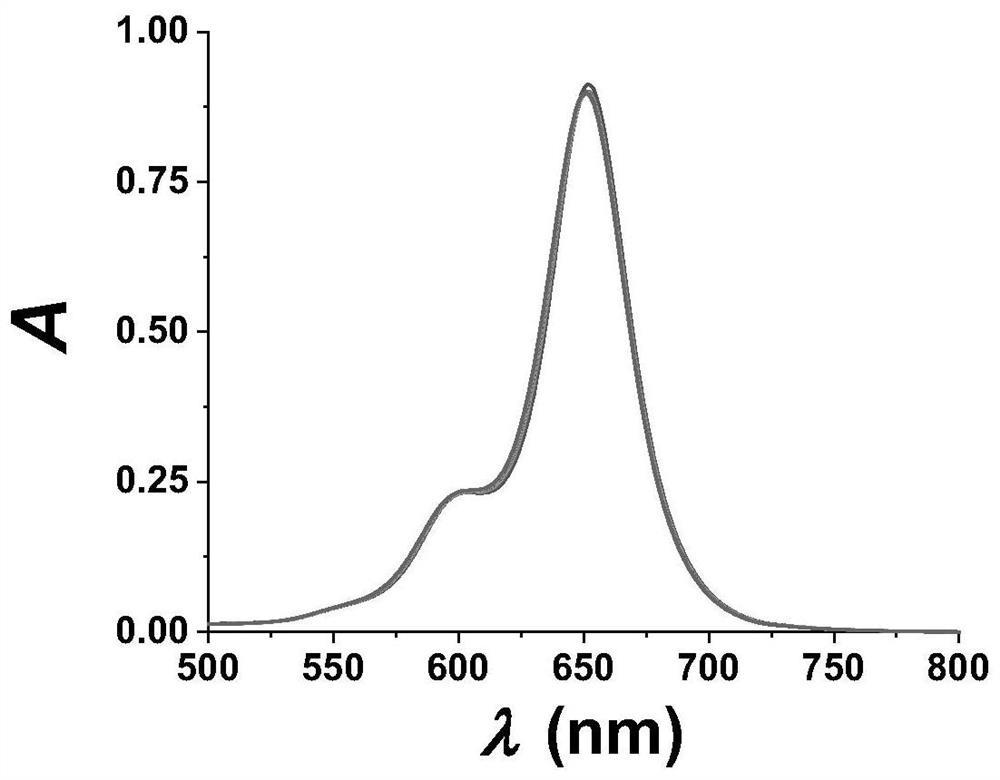
Fluorescent probe for specifically responding to hypochlorous acid based on BODIPY dye and preparation and application
A fluorescent probe, hypochlorous acid technology, applied in the field of fluorescent probes, can solve the problems of short fluorescence emission wavelength, low selectivity, lack of imaging applications, etc., and achieve the effects of simple synthesis method, obvious detection signal, and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation steps of the probe are as follows:
[0042] (1) Preparation of compound 1
[0043]
[0044] N 2Under protection, dissolve p-hydroxybenzyl alcohol in 1,2-dichloroethane, add diisopropylethylamine in an ice-water bath, and add N,N-dimethylaminothioformyl chloride in a ratio of 1:1 equivalent , heated at 50°C for 24h, extracted with dichloromethane, and separated by column chromatography to obtain compound 1;
[0045] (2) Preparation of Compound 2
[0046]
[0047] The compound 1 prepared in step (1) was dissolved in dichloromethane, phosphorus tribromide was added in a ratio of 1:1.2 equivalent, stirred at room temperature for 1-2 hours, quenched with water, extracted with dichloromethane, and separated by column chromatography to obtain Compound 2;
[0048] (3) Preparation of compound 3
[0049]
[0050] N 2 Under the protection of the ice-water bath, sodium hydride is added to the tetrahydrofuran solvent and mixed to obtain a mixed solvent...
Embodiment 2
[0060] The preparation steps of the probe are as follows:
[0061] (1) Preparation of compound 1
[0062] N 2 Under protection, dissolve p-hydroxybenzyl alcohol in 1,2-dichloroethane, add diisopropylethylamine in an ice-water bath, and add N,N-dimethylaminothioformyl chloride at an equivalent ratio of 1:2 , heated at 50°C for 24h, extracted with dichloromethane, and separated by column chromatography to obtain compound 1;
[0063] (2) Preparation of Compound 2
[0064] The compound 1 prepared in step (1) was dissolved in dichloromethane, phosphorus tribromide was added in a ratio of 1:1 equivalent, stirred at room temperature for 1~2h, quenched with water, extracted with dichloromethane, and separated by column chromatography to obtain Compound 2;
[0065] (3) Preparation of compound 3
[0066] N 2 Under the protection of the ice-water bath, sodium hydride is added to the tetrahydrofuran solvent and mixed to obtain a mixed solvent, and acetonyl p-toluenesulfonate oxime e...
Embodiment 3
[0072] The preparation steps of the probe are as follows:
[0073] (1) Preparation of compound 1
[0074] N 2 Under protection, dissolve p-hydroxybenzyl alcohol in 1,2-dichloroethane, add N,N-dimethylaminothioformyl chloride at an equivalent ratio of 1:1.5, and add diisopropylethylamine in an ice-water bath , heated at 50°C for 24h, extracted with dichloromethane, and separated by column chromatography to obtain compound 1;
[0075] (2) Preparation of Compound 2
[0076] The compound 1 prepared in step (1) was dissolved in dichloromethane, phosphorus tribromide was added according to the ratio of 1:2 equivalent, stirred at room temperature for 1-2h after adding alkali, quenched with water, extracted with dichloromethane, column layer Analysis and separation obtained compound 2;
[0077] (3) Preparation of compound 3
[0078] N 2 Under the protection of the ice-water bath, sodium hydride is added to the tetrahydrofuran solvent and mixed to obtain a mixed solvent, and acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com