Amphiphilic phosphorus-containing crown macromolecular material as well as preparation and application thereof
A macromolecular and amphiphilic technology, applied in medical preparations containing active ingredients, organic active ingredients, medical preparations with non-active ingredients, etc., to achieve good water solubility and biocompatibility, low cost, and good development foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
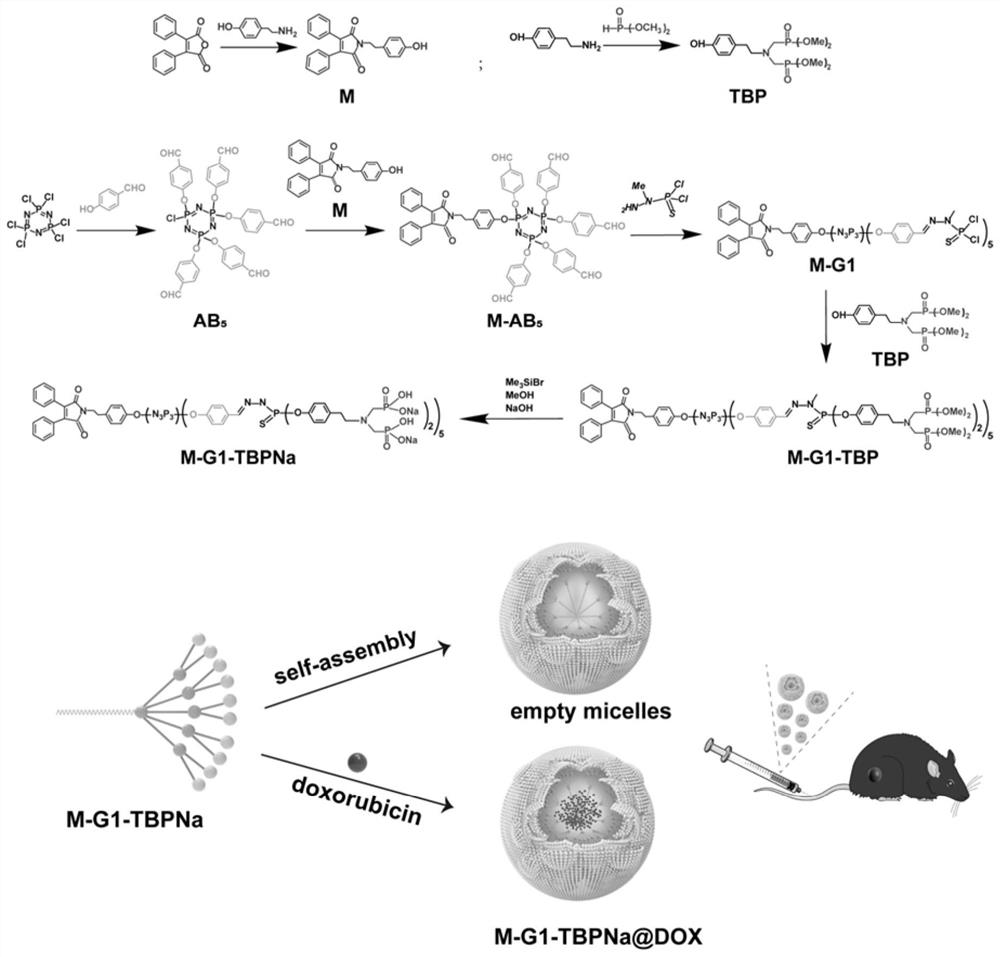
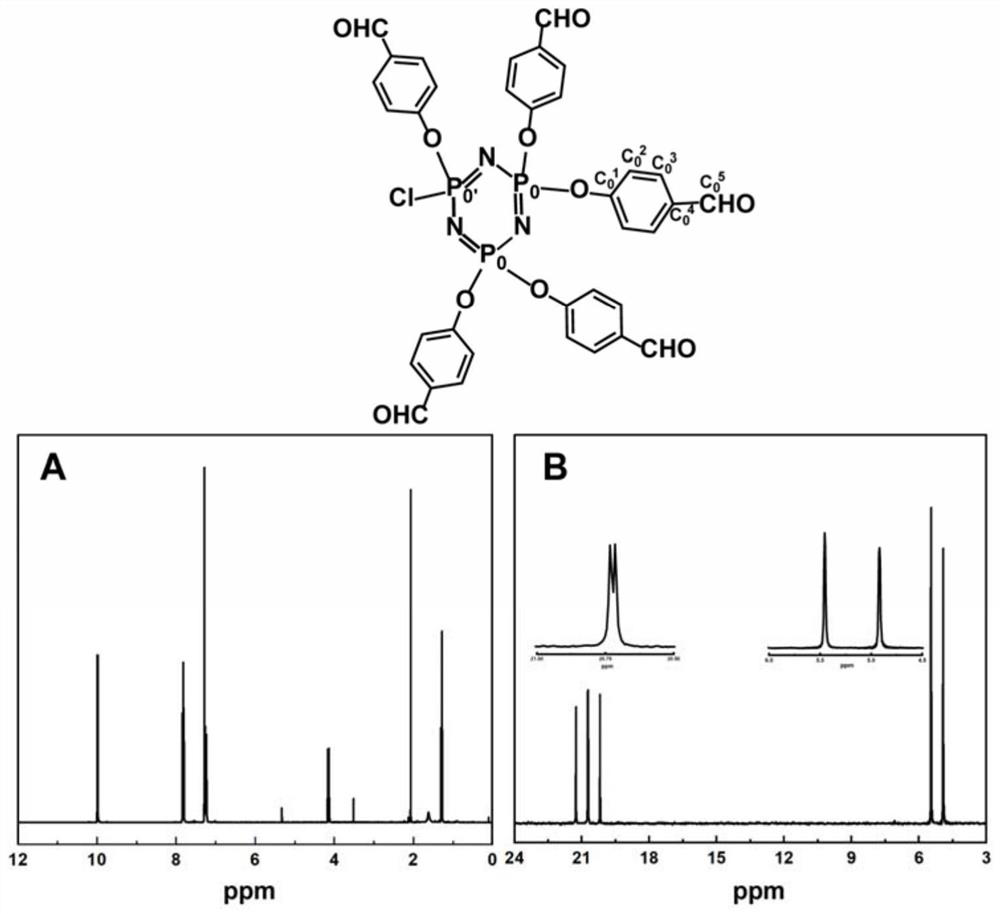
[0097] (1) Put hexachlorocyclotriphosphazene (2.26mmol, 786mg) and anhydrous cesium carbonate (13.56mmol, 4.418g) into a reaction flask containing anhydrous THF (40mL), ice bath, dropwise add 4- Hydroxybenzaldehyde (11.3 mmol, 1.38 g). Subsequently, the ice bath was removed, and the mixture was stirred overnight at room temperature. After the reaction, it was centrifuged and concentrated under reduced pressure to a transparent solution, followed by purification by silica gel column chromatography (pentane / ethyl acetate), and finally obtained a colorless oily AB with 76% yield. 5 .
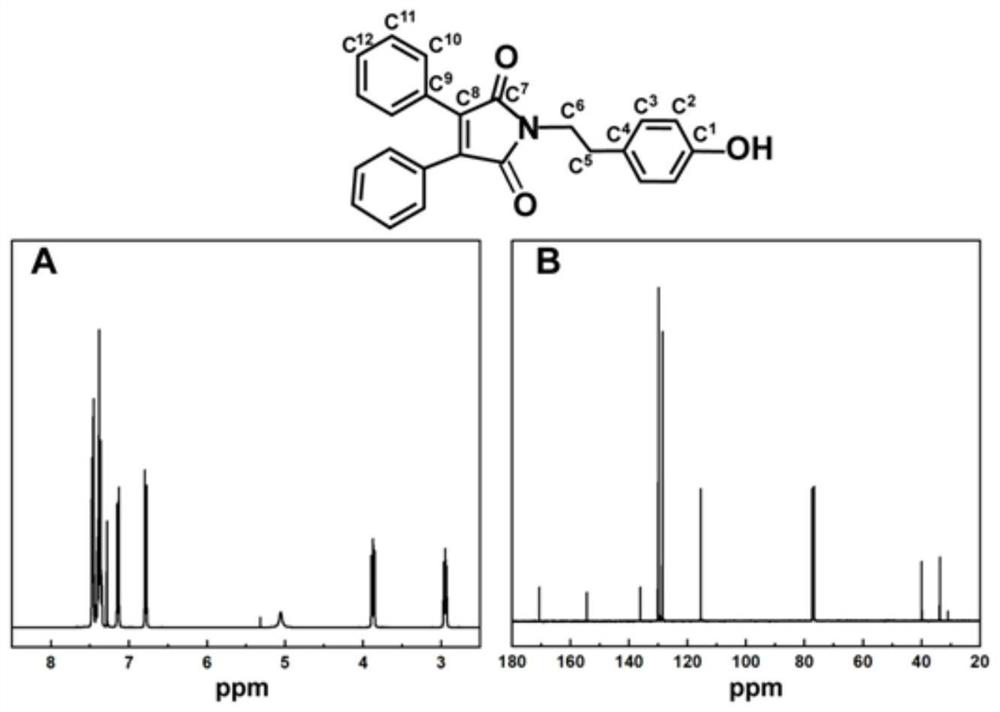
[0098] (2) Dissolve 2,3-diphenylmaleic anhydride (4mmol, 1g) and tyramide (4mmol, 0.548g) in acetic acid, and add N,N-diisopropyl dropwise at 160°C-170°C Ethylamine (DAPIPEA) solution, reacted for 90 minutes. After the above substance was cooled to room temperature, 50 mL of distilled water was added and filtered to obtain a yellow precipitate. Purified by column chromatography (the mobile phas...
Embodiment 2
[0136] Prepare different concentrations of M-G1-TBPNa solutions (0.001mg / mL-2mg / mL), and add the prepared solutions to the solution containing the fluorescent probe pyrene (10μL, 4.0×10 -4 M) in the volumetric flask, the final concentration of pyrene is 6.0 × 10 -7 M, sonicate for 30 minutes, store overnight at room temperature. The fluorescence spectrophotometer measures the fluorescence spectrum with an excitation wavelength of 333nm, sets the incident slit width of the steady-state fluorometer to 1.0nm, and the receiving slit width to 1.2nm, and records the fluorescence curve of each solution in the range of 350-435nm. The lg value and I of M-G1-TBPNa concentration 373 / I 393 The fluorescence intensity ratio is analyzed on the horizontal and vertical coordinates, such as Figure 9 As shown, with the increase of M-G1-TBPNa concentration I 373 / I 393 The fluorescence intensity ratio of the first increase, followed by a significant decline at 208.5μM. This shows that the ...
Embodiment 3
[0138] Dissolve doxorubicin hydrochloride (DOX) in methanol, then add triethylamine (TEA) in an equimolar amount to doxorubicin hydrochloride, and ultrasonicate for 5 minutes to deprotonate doxorubicin hydrochloride into doxorubicin. According to different molar ratios (M-G1-TBPNa:DOX=1:0.5, 1:1, 1:2), add a certain amount of DOX methanol solution into the M-G1-TBPNa aqueous solution, and stir at room temperature overnight in the dark . Then move the mixed solution into a centrifuge tube, and centrifuge twice at 7000rpm for 20 minutes. After each centrifugation, take the supernatant, resuspend the precipitate with an appropriate amount of ultrapure water, and then perform the next centrifugation. Dissolve the precipitate in 1 mL of methanol, measure its UV absorbance at 480 nm, and calculate the encapsulation and uploading efficiencies of DOX by comparing with the standard curve of pure DOX. The results are shown in Table 1. When the feed ratio of M-G1-TBPNa and DOX was 1:0.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com