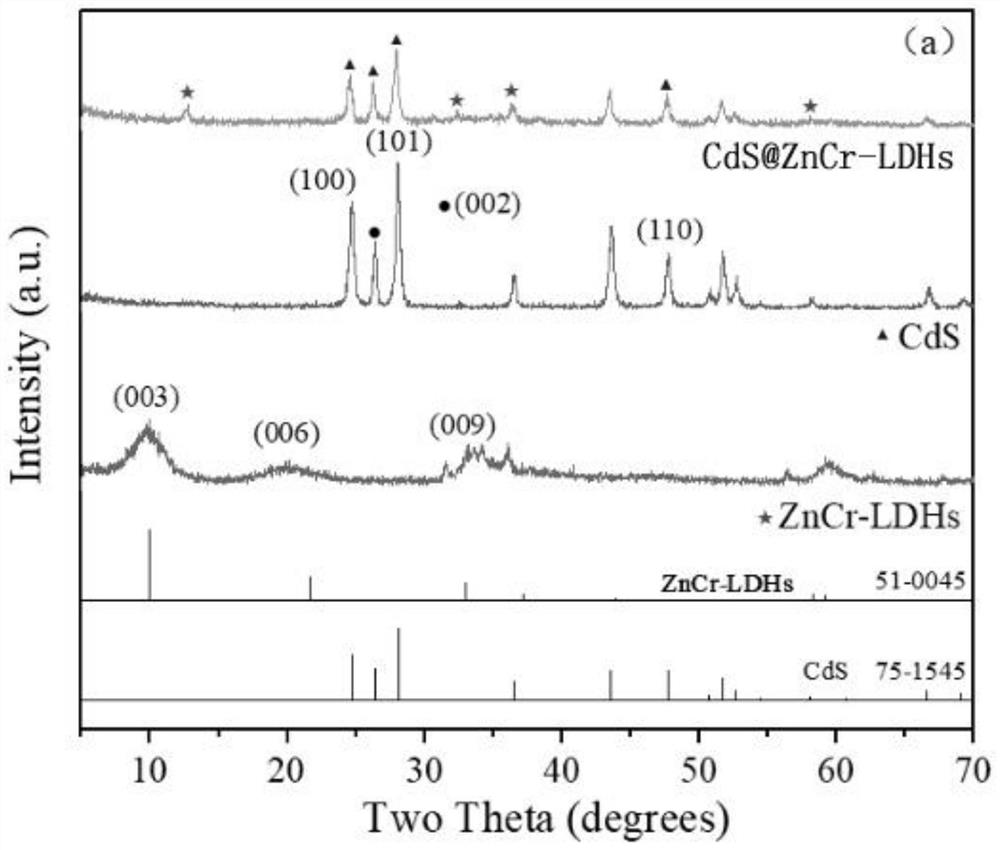
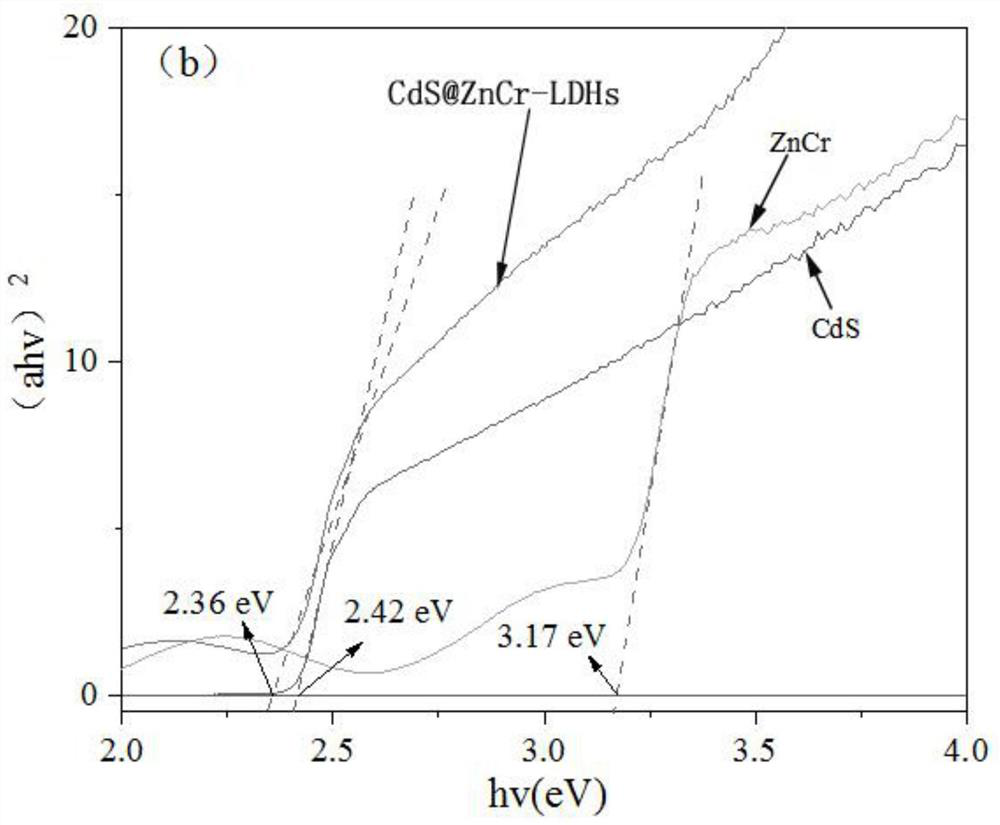
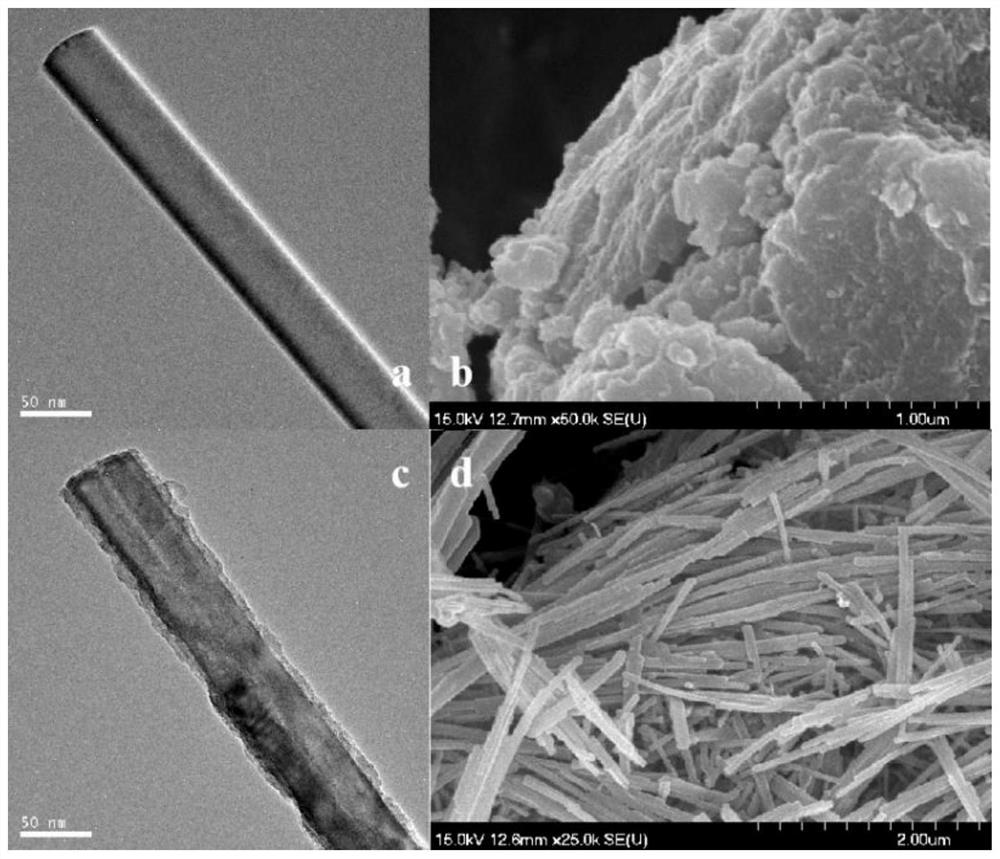
CdS@ZnCr-LDHs heterojunction nanomaterial for photocatalytic degradation of tetracycline as well as preparation method and application thereof
A nano-material and tetracycline technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as easy recombination of CdS, photocorrosion, and constraints, and achieve Effect of improving photocatalytic performance, strong thermal stability and photochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1Z
[0020] The synthesis of embodiment 1ZnCr-LDHs material
[0021] The details are as follows: under nitrogen protection atmosphere, 0.075mol (22.31g) Zn(NO 3 ) 2 ·6H 2 O and 0.025mol (10.00g) Cr(NO 3 ) 3 9H 2 O was dissolved in 100ml deionized water to prepare solution A. Solution B is 1M sodium hydroxide solution. In a three-necked flask with 50ml of deionized water, the liquid in the three-necked flask was stirred at room temperature (rotating speed was 200r / min), and solution A and solution B were all dripped drop by drop in the three-necked flask, and the three-necked flask was controlled. The pH value of the internal mixture is 9.0-9.5. After the solution A is added dropwise within 30 minutes, the dropwise addition of solution B is also stopped. The resulting mixture was crystallized at 65 °C for 24 h, centrifuged and washed until neutral, and dried in a vacuum at 85 °C for 12 h to finally obtain the sample ZnCr-NO 3 -LDHs, recorded as ZnCr-LDHs material.
Embodiment 2
[0022] The synthesis of embodiment 2CdS
[0023] The details are as follows: the synthesis of CdS by hydrothermal method mainly uses Cd(NO 3 ) 2 4H 2 O, thiourea and ethylenediamine are raw materials, and the synthesis process is as follows: at room temperature, 0.0197mol (6.0768g) Cd(NO 3 ) 2 4H 2 The thiourea of 0 and 0.0591mol (4.5g) is dissolved in 60ml ethylenediamine, stirs and transfers in the 100ml polytetrafluoroethylene reactor, airtight polytetrafluoroethylene reactor, then the polytetrafluoroethylene reactor is transferred to React in a vacuum oven at 160°C for 48 hours, then cool naturally to room temperature, centrifuge the yellow precipitate in the reaction solution, wash the yellow precipitate twice with deionized water and ethanol, and dry at 65°C for 10 hours to obtain nano-CdS products .
Embodiment 3
[0024] Synthesis of Example 3CdS@ZnCr-LDHs composite material
[0025] In the synthesis of CdS@ZnCr-LDHs materials, overboiling water was used to avoid affecting the dissolved carbon dioxide, as follows: 0.007mol (100mg) cadmium sulfide (CdS), 0.0147mol (4.373g) Zn(NO 3 ) 2 ·6H 2 O, 0.0049mol (1.961g) Cr(NO 3) 3 9H 2 O, and 0.037mol (2.248g) of urea were dissolved in 100ml of deionized water, the resulting solution was ultrasonically treated for 15min, and then stirred for 30min to disperse the reaction system evenly. Transfer the above solution to a polytetrafluoroethylene reaction kettle, seal the polytetrafluoroethylene reaction kettle, then place the polytetrafluoroethylene reaction kettle in a 95°C oven for 12 hours to react, take it out, cool it down to room temperature naturally, and put the reaction The solution was centrifuged, and the obtained precipitated solid was washed twice with deionized water and absolute ethanol, respectively. After vacuum drying at 50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com