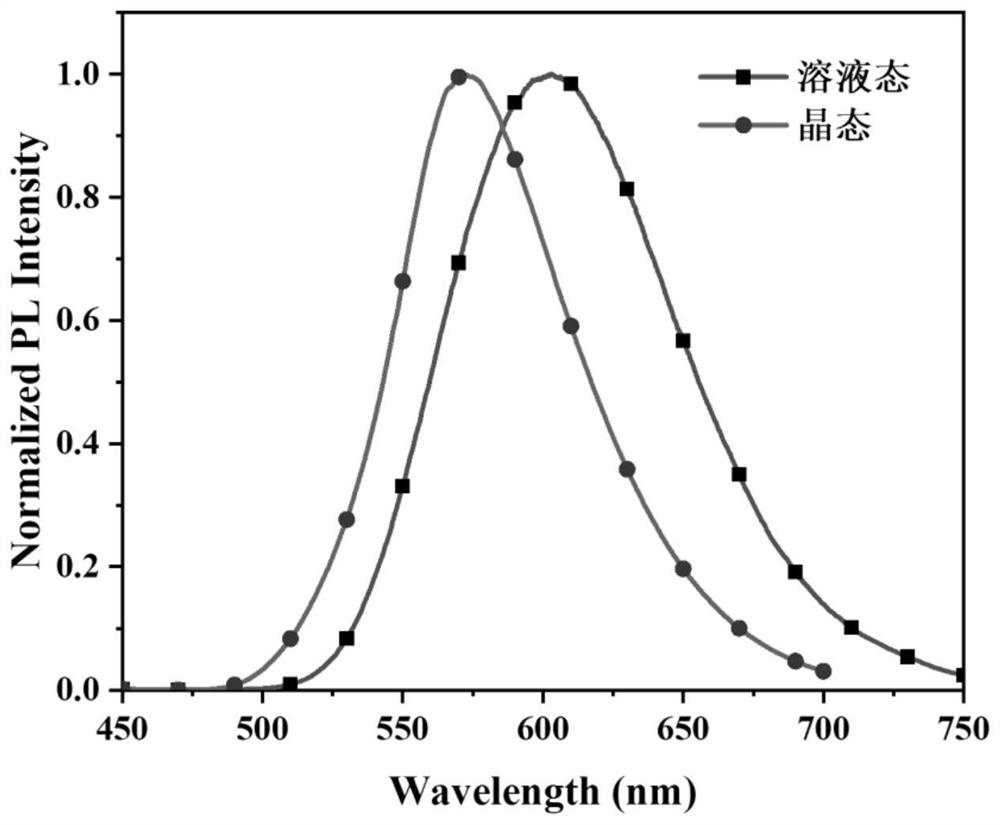
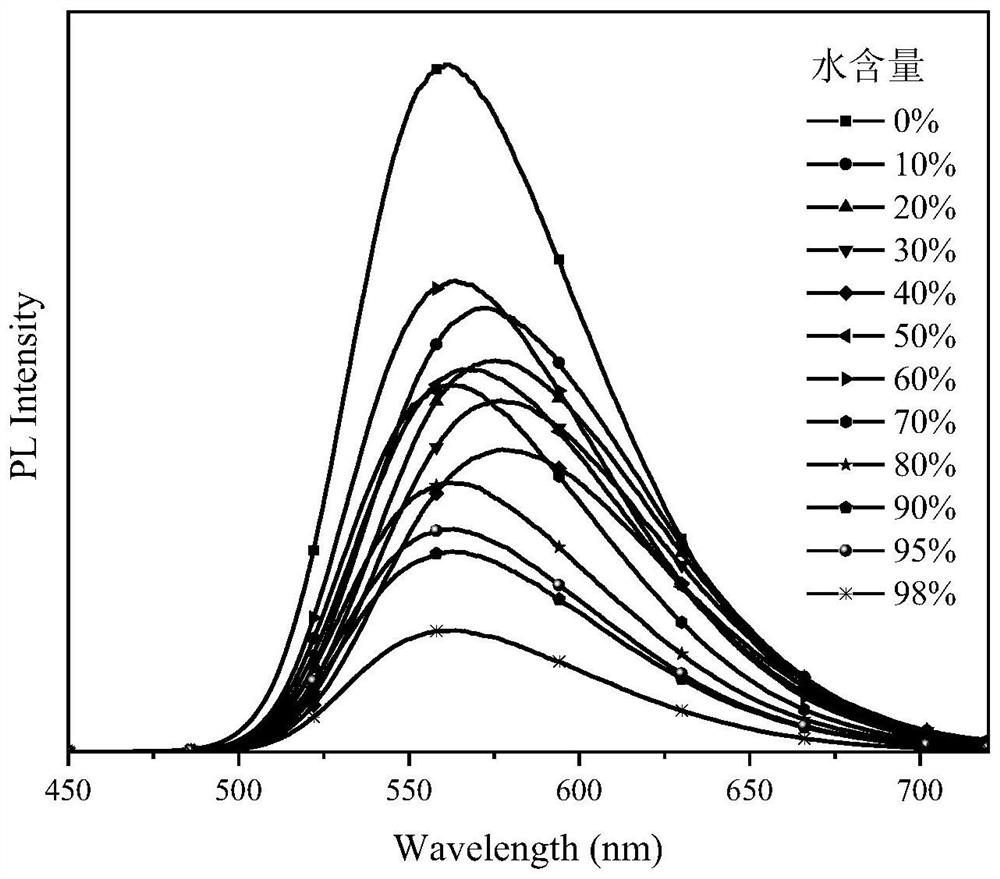
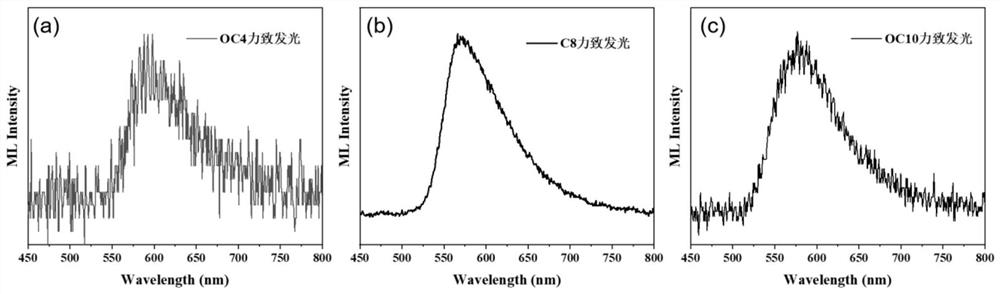
Small molecular material with yellow mechanoluminescence of 10-membered fused ring benzothiadiazole as well as preparation method and application of small molecular material
A benzothiadiazole and luminescent technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., to achieve the effect of small interference and sensitive brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Synthesis of compound C4
[0075] The synthetic route is as follows:
[0076]
[0077] The preparation method of C4, its main steps are:
[0078] 1) 4,8-diketone-benzo[1,2-b:4,5-b']dithiophene (5.0g, 22.7mmol) was dissolved in 50mL ethanol, and sodium borohydride (3.4 g, 90.8 mmol), after the addition, react in an ice bath at room temperature for 5 hours; cool to room temperature, slowly add the reaction solution into dilute hydrochloric acid, collect the filter cake by filtration, and dry in vacuo to obtain 4.9 g of a light green solid. Yield 98%. This step is directly carried out to the next reaction without purification.
[0079] 2) Under an inert atmosphere, compound 2 (2.0g, 9.0mmol), n-butane bromide (3.1g, 22.5mmol), potassium carbonate (2.5g, 18.0mmol) and potassium iodide (0.30g, 1.80mmol) were dissolved in In 20mL N,N dimethylformamide, react at 90°C for 8 hours. Cool to room temperature, collect the filtrate by filtration, concentrate the filtrate and...
Embodiment 2
[0085] Synthesis of compound C8
[0086] The synthetic route is as follows:
[0087]
[0088] The preparation method of C8, its main steps are:
[0089] 1) The synthesis of compound 2 is the same as that of C4 in Example 1. This step is directly carried out to the next reaction without purification.
[0090] 2) Under an inert atmosphere, compound 2 (2.0g, 9.0mmol), n-octane bromide (4.35g, 22.5mmol), potassium carbonate (2.49g, 18.0mmol) and potassium iodide (0.30g, 1.80mmol) were dissolved in In 20mL N,N dimethylformamide, react at 90°C for 8 hours. Cool to room temperature, collect the filtrate by filtration, concentrate the filtrate and dilute with dichloromethane, then add water and dichloromethane for extraction and separation, collect the organic phase and dry it with anhydrous sodium sulfate. Use a silica gel column for separation, and the eluent is petroleum ether:dichloromethane=8:1. After drying in vacuum, 3.5 g of white powdery solid was obtained with a yiel...
Embodiment 3
[0096] Synthesis of Compound C10
[0097] The synthetic route is as follows:
[0098]
[0099] The preparation method of C10, its main steps are:
[0100] 1) The synthesis of compound 2 is the same as that of C4 in Example 1. This step is directly carried out to the next reaction without purification.
[0101] 2) Under an inert atmosphere, compound 2 (2.0g, 9.0mmol), n-bromodecane (4.98g, 22.5mmol), potassium carbonate (2.49g, 18.0mmol) and potassium iodide (0.30g, 1.80mmol) were dissolved in In 20mL N,N dimethylformamide, react at 90°C for 8 hours. Cool to room temperature, collect the filtrate by filtration, concentrate the filtrate and dilute with dichloromethane, then add water and dichloromethane for extraction and separation, collect the organic phase and dry it with anhydrous sodium sulfate. Use a silica gel column for separation, and the eluent is petroleum ether:dichloromethane=8:1. After vacuum drying, 3.62 g of a white powdery solid was obtained with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com