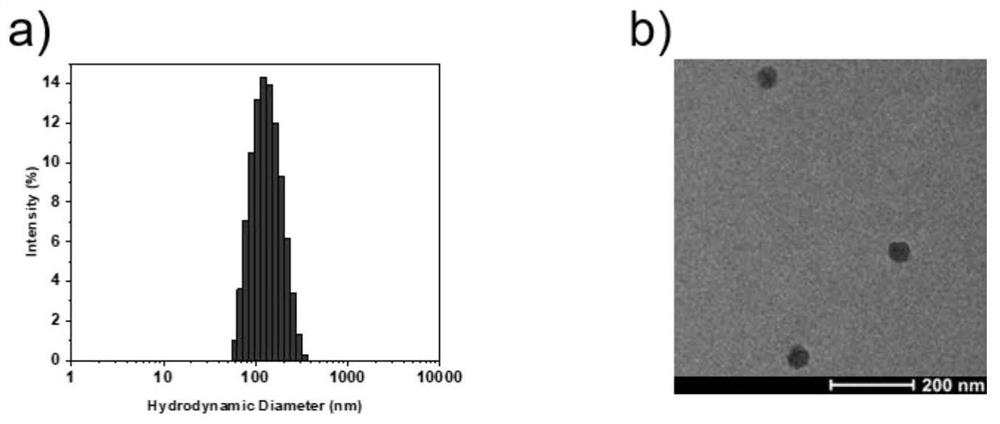
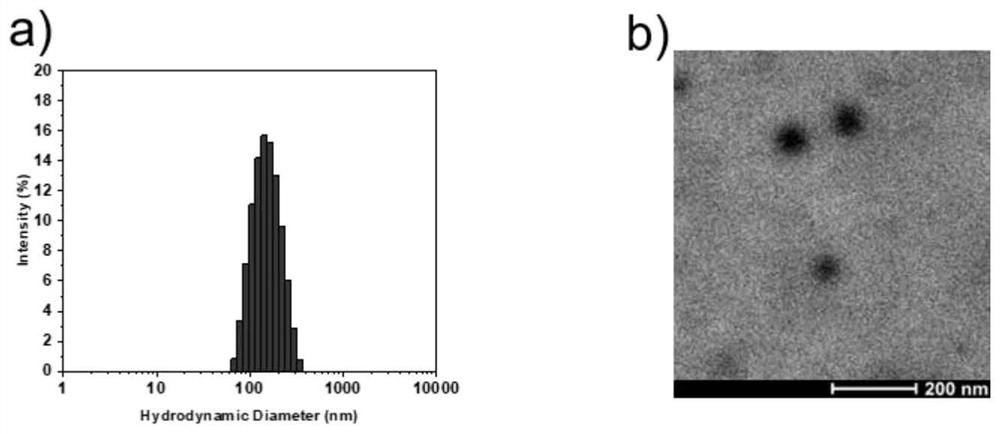
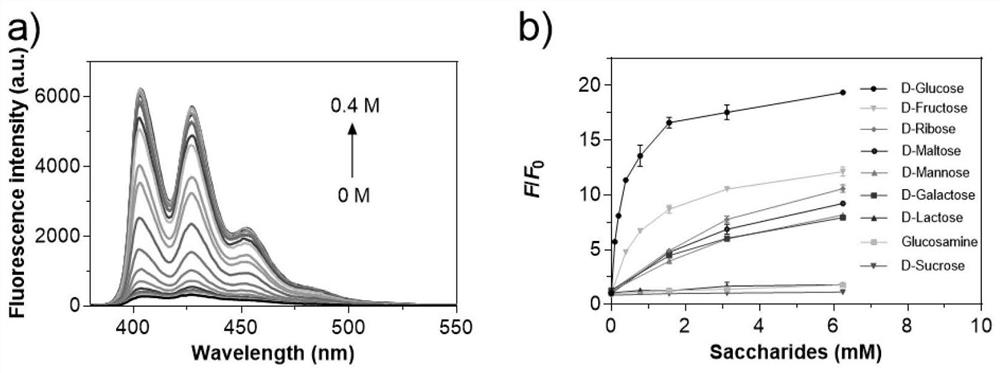
Liposomal glucose fluorescent probe with good biocompatibility and preparation method thereof
A biocompatible, fluorescent probe technology, applied in the field of fluorescence analysis and detection, can solve the problems of low sensitivity and selectivity, low water solubility, and long response time, and achieve high affinity and selectivity, low toxicity, and good solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis of molecular probe CN-DBA, its synthetic route is as follows:
[0046]
[0047] 1) Synthesis of compound C2:
[0048] Take a 500mL round-bottom flask, add 4-cyano-2-methylphenylboronic acid (5.00g, 31.06mmol), neopentyl glycol (3.88g, 37.25mmol) and 200mL toluene, add a Dean-Stark trap, Then the reaction was refluxed for 20 h, and the reaction was detected by TLC. After the reaction was completed, the reaction solvent was removed by drying under reduced pressure. The crude product was dissolved in dichloromethane and purified by flash column chromatography using dichloromethane as the eluent to obtain 6.76 g of oily compound C2 with a yield of 95.0%. 1 H NMR(400MHz, Chloroform-d)δ7.79(d,J=7.5Hz,1H),7.40(d,J=8.0Hz,2H),3.78(s,4H),2.52(s,3H),1.04 (s,6H).
[0049] 2) Synthesis of compound C3:
[0050] Take a 500mL round bottom flask, add compound C2 (6.76g, 29.51mmol), N-bromosuccinimide (5.52g, 30.99mmol), AIBN (0.13g, 0.79mmol) and carbon tetrachlorid...
Embodiment 2
[0058] The synthesis of molecular probe Ac-CDBA, its synthetic route is as follows:
[0059]
[0060] Synthesis steps:
[0061] 1) Synthesis of compound C7:
[0062] Take a 250mL round bottom flask, add aluminum chloride (2.61g, 19.57mmol), 9,10-dimethylanthracene (2.79g, 13.52mmol), anhydrous acetyl chloride (1.5mL, 21.1mmol) and 150mL carbon disulfide, room temperature Stir for 12h. Then the reaction system was heated to 45 °C for 2 h. The reaction was detected by TLC. After the reaction was completed, 45 mL of ice water containing 2.4 mL of hydrochloric acid was added, the reaction system was cooled to room temperature, the organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, filtered, and the reaction solvent was removed under reduced pressure. Dichloromethane was used as the eluent for purification by flash column chromatography to obtain 1.59 g of compound C-8 with a yield of 47.3%. 1 H NMR (400MHz, Chloroform-d) δ 9.00 (d, J=1.5H...
Embodiment 3
[0070] The liposomal glucose fluorescent probe NPs- CN-DBA and NPs- Ac-CDBA The preparation method is as follows:
[0071] 1) Preparation of liposome fluorescent probe NPs by ultrasonic method- CN-DBA . First, CN-DBA (2.91 mg) prepared in Example 1 and DSPE-PEG were 2000 (14.55mg, DSPE-PEG 2000 and CN-DBA in a mass ratio of 5:1) dissolved in 1 mL of methanol. Under the ultrasonic condition of a cell sonicator (XL2000, Misonix Incorporated, NY), the above methanol mixed solution was injected into deionized water (15 mL) using a syringe (1 mL), and the mixed solution was continuously sonicated for 15 s. Then, the mixed solution was taken out and transferred to a regenerated cellulose dialysis bag with a molecular weight cut-off of 3500 (MW Cut-off: 3500) for dialysis for 24 hours. The dialyzed solution was concentrated to a certain volume using an ultrafiltration tube, and finally the liposome fluorescent probe NPs- CN-DBA .
[0072] 2) Preparation of liposome fluorescen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com