Method for synthesizing ammonia through electro-catalysis of nitrate or nitrite
A technology of nitrite and nitrate, applied in the field of electrocatalysis, can solve the problems of poor structural stability, poor selectivity, and overpotential, etc., and achieves the effects of stable structure, simple preparation process, and high-efficiency electrocatalysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Commercially purchased copper foam with a thickness of 0.5 cm, a width of 10 cm, and a length of 10 cm was ultrasonically cleaned in water, acetone, and ethanol for 30 min, respectively, and then dried. Then heat for a certain period of time in an air atmosphere at 80° C. to obtain a foamed copper-copper oxide catalytic electrode, and the content of copper oxide is controlled by the heating time. In this embodiment, the heating time is 40 minutes, and copper oxide accounts for 10%.
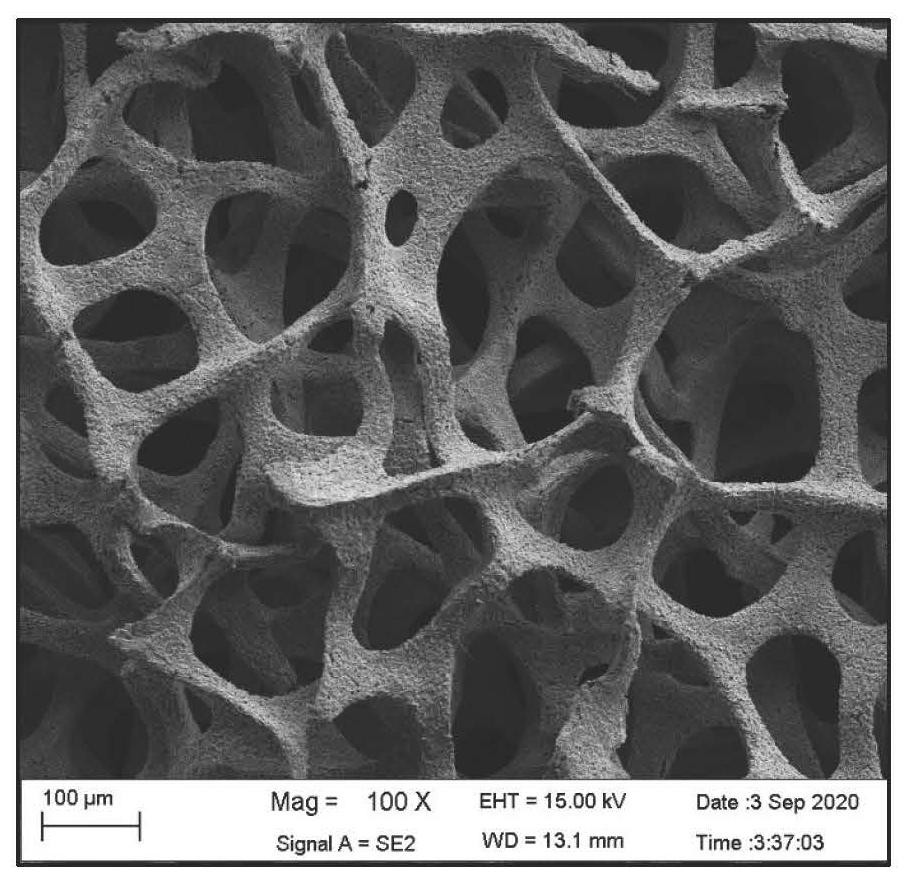
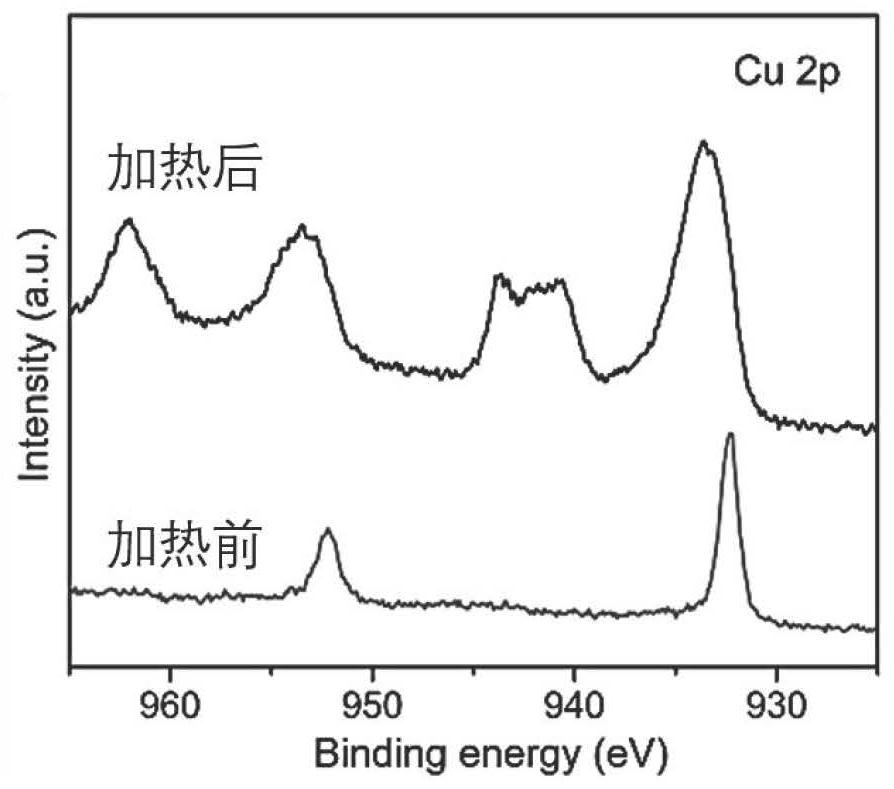
[0045] figure 1 It is a scanning electron micrograph of foamy copper-copper oxide. figure 2 The XPS ray energy spectrum confirmed that copper oxide was produced after heating.
[0046] The catalytic electrode cut according to the size of 2cm in length and width is used as the working electrode (cathode). In the three-electrode (counter electrode: nickel foam; reference electrode: Ag / AgCl electrode) system, the electrolyte is: 0.5mmol / L Aqueous solution of potassium nitrate. In the atmosp...
Embodiment 2
[0049] Replace the aqueous solution of 0.5mmol / L potassium nitrate with 0.1mol / L potassium nitrate and 0.1mol / L sodium nitrite aqueous solution of higher concentration, all the other are with embodiment 1. In the atmospheric environment, apply -0.15V (vs RHE) and test for 1800s.
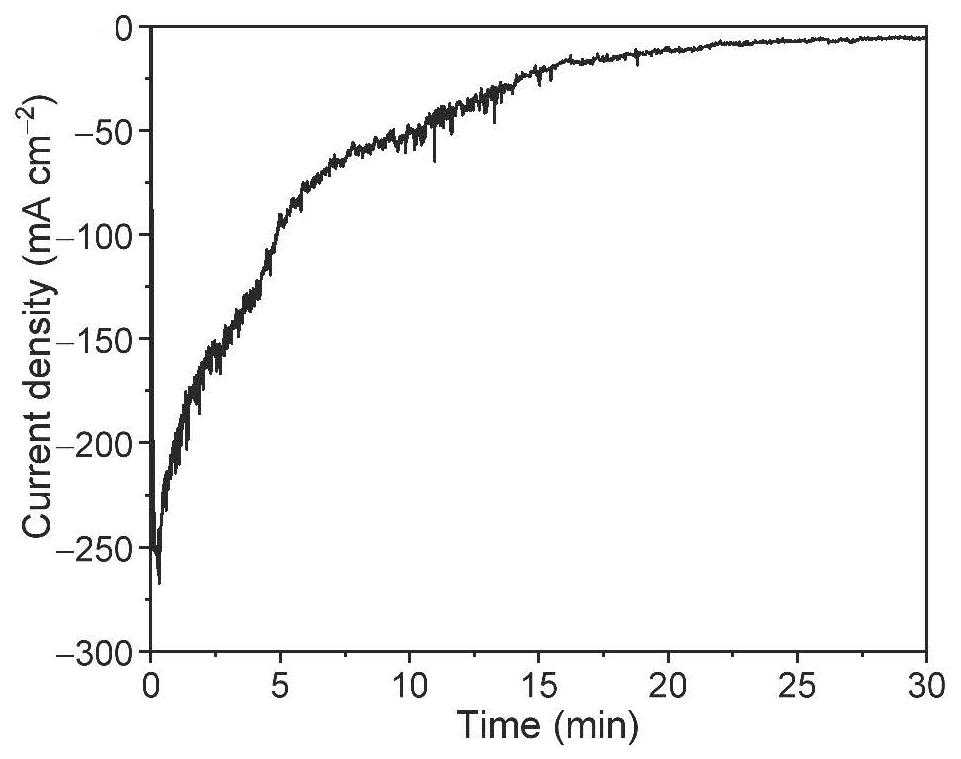
[0050] Figure 5 It is the obtained i-t diagram, which shows that during the test, the catalytic electrode can obtain a relatively stable and high ammonia production current. The ammonia concentration in the electrolyte was tested by spectrophotometry, and the ammonia production rate was analyzed to be 1.24mmol h -1 cm -2 , the selectivity of electrochemical ammonia production is 98.9%.
Embodiment 3
[0052] Replace copper foam with metal copper sheet, all the other are the same as embodiment 1. In the atmospheric environment, apply -0.15V (vs RHE) to test for 1800s, Figure 6 In order to obtain current-time (i-t) and Figure 7 The graph (c-t) of corresponding ammonia and nitrite concentration and time shows that the current decreases with the decrease of nitrate concentration during the test, and the rate of ammonia production after analysis is 1.12mmolh -1 cm -2 , the selectivity of electrochemical ammonia production is 93.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com