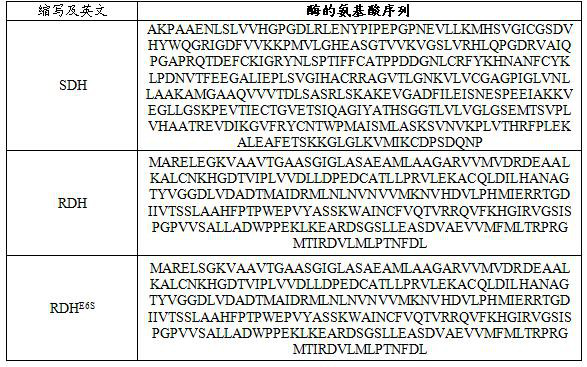
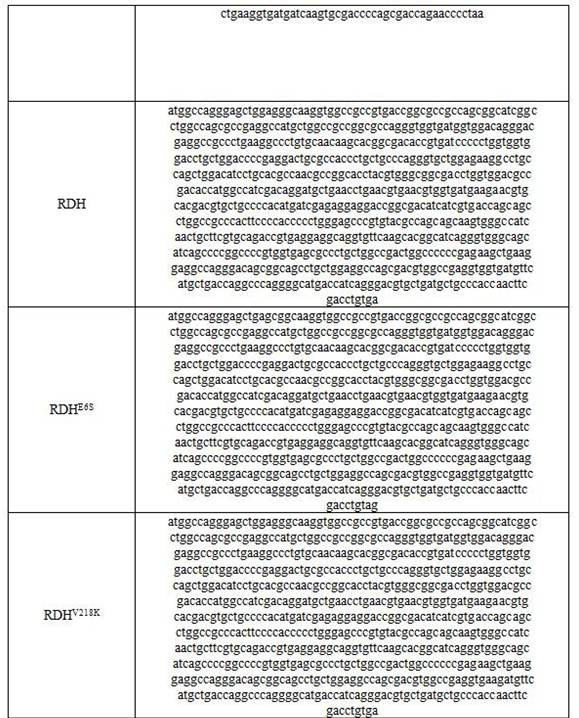
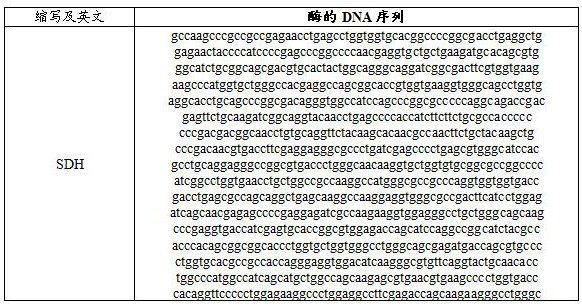
Enzyme composition and method for synthesizing Pro-xylane by chemical enzyme method
A chemical enzymatic method and bosine technology, which can be used in biochemical equipment and methods, botanical equipment and methods, and plant genetic improvement, etc., and can solve the problems of poor carbonyl stereoselectivity of chemical reductone, pollutants, and difficulty in separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Preparation of β-acetone xyloside 2.
[0084] Add 150 ml of methanol, 31.8 g (0.3 mol) of sodium carbonate, 30 g (0.2 mol) of xylose, and 26 g (0.24 mol) of acetylacetone to a 500 ml reaction flask in sequence. The pH was adjusted to 7 with HCl, and the crude β-acetone xyloside 2 was obtained by spin-drying. MS (ESI): m / z: 191.14 [M+H] + .
Embodiment 2
[0085] Example 2: SDH catalytic reduction of β-acetone xyloside 2 to prepare Boseine
[0086] In 1 L of 50 mM pH7.4 sodium phosphate buffer solution, 50 g of the crude β-acetone xyloside 2 obtained in Example 1, 3.0 g of β-nicotinamide adenine dinucleotide phosphate (NADP) were added successively. + ) monosodium salt (0.4 mM), 52 g sodium phosphite pentahydrate (240 mM), and 50 ml isopropanol. The crude solution (4000U SDH and 6000U PTDH) was added at a constant temperature of 30~40°C, and the reaction was slowly stirred at 30°C for 6 hours. After the reaction was completed, centrifuge, the supernatant was filtered to remove salt, and the water was evaporated under reduced pressure to obtain 45 g of the product, MS (ESI): m / z: 193.01 [M+H] + .
[0087] The Boseine obtained in Example 2 was analyzed by nuclear magnetic resonance, and the results were obtained 1 H NMR (400 MHz, D 2 O) δ 4.94 – 4.86 (m, 3H), 4.25 (d, J = 4.3 Hz, 1H), 3.84 – 3.73 (m, 1H), 3.67-3.63 (m, 1H), ...
Embodiment 3
[0088] Example 3: Wild-type RDH enzyme catalyzed reduction of β-acetone xyloside 2 to prepare boson
[0089] In 1L of 50 mM pH7.4 sodium phosphate buffer solution, 50 g of the crude β-acetone xyloside 2 obtained in Example 1, 3.0 g of β-nicotinamide adenine dinucleotide phosphate (NADP) were added successively. + ) monosodium salt (0.4 mM), 52 g sodium phosphite pentahydrate (240 mM), and 50 ml isopropanol. The crude solution (4000U wild-type RDH and 6000U PTDH) was added at a constant temperature of 30 to 40°C, and the reaction was slowly stirred at 30°C for 6 hours. After the reaction, centrifugation was performed, and the supernatant was nanofiltered to remove salt, evaporated under reduced pressure to remove water, and purified to obtain 35.1 grams. Boseine, purity 98.2%, yield 91.4%.
[0090] The boson obtained in Example 3 was analyzed by mass spectrometry, and the result was obtained MS (ESI): m / z: 215.01 [M+Na] + .
[0091] The Boseine obtained in Example 3 was anal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com