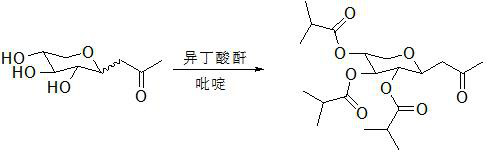
Method for synthesizing Pro-Xylane through acylation protection and reduction
A Boseine and reducing agent technology, which is applied in the field of Boseine synthesis through acylation protection and reduction, can solve the problems of difficult mass production and difficult removal, and achieve the effect of high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
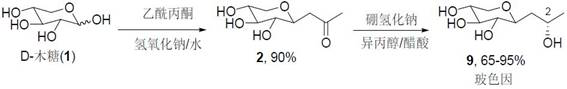
[0054] Add 150 ml of methanol, 31.8 g (0.3 mol) of sodium carbonate, 30 g (0.2 mol) of xylose, and 26 g (0.24 mol) of acetylacetone to a 500 ml reaction flask in sequence. After reacting for 12 hours at 80 degrees Celsius, use Adjust the pH to 7 with 4N HCl, spin dry to obtain the crude product of β-acetone xyloside, MS (ESI): m / z: 191.07[M+H] + .
Embodiment 2
[0056]
[0057] All the crude β-acetone xyloside obtained in Example 1 was added into a 500 ml reaction flask, followed by adding 150 ml of pyridine and 24.7 g (0.24 mol) of acetic anhydride, and reacting at 25 degrees Celsius for 24 hours. Add 200 ml of ethyl acetate to dilute, extract with 1 mole per ml of hydrochloric acid solution, use 200 ml each time, extract three times, then extract once with 200 ml of saturated sodium bicarbonate solution, extract once with 100 ml of saturated saline, extract once with 10 g Dry the organic phase with anhydrous sodium sulfate, then add 1.5 g of activated carbon and stir at 50°C for 30 minutes to decolorize, filter with suction, and spin the filtrate to obtain 54.1 g of crude product.
[0058] Use petroleum ether and ethyl acetate in a ratio of 5:2 to prepare 150 ml of a mixed solution, add it to a 500 ml flask containing the crude product, heat and reflux at 60 degrees Celsius and stir, and after it is completely dissolved, slowly co...
Embodiment 3
[0060]
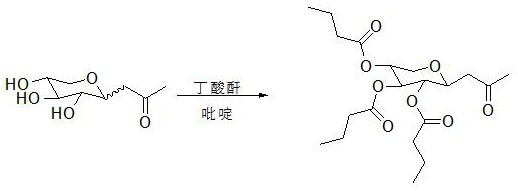
[0061] All the crude β-acetone xyloside obtained in Example 1 was added into a 500 ml reaction flask, 150 ml of pyridine was added, and 33.6 g (0.24 mol) of benzoyl chloride was added dropwise under an ice bath, and the reaction was carried out at 25 degrees Celsius for 24 hours. Add 200 ml of ethyl acetate to dilute, extract with 1 mole per ml of hydrochloric acid solution, use 200 ml each time, extract three times, then extract once with 200 ml of saturated sodium bicarbonate solution, extract once with 100 ml of saturated saline, extract once with 10 g Dry the organic phase with anhydrous sodium sulfate, then add 1.5 g of activated carbon, stir at 50°C for 30 minutes to decolorize, filter with suction, and spin the filtrate to obtain 91.5 g of crude product.
[0062] Use petroleum ether and ethyl acetate in a ratio of 5:2 to prepare 150 ml of a mixed solution, add it to a 500 ml flask containing the crude product, heat and reflux at 60 degrees Celsius and stir, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com